Hepatic palmitoyl-proteomes and acyl-protein thioesterase protein proximity networks link lipid modification and mitochondria
- PMID: 37925639
- PMCID: PMC10872372
- DOI: 10.1016/j.celrep.2023.113389
Hepatic palmitoyl-proteomes and acyl-protein thioesterase protein proximity networks link lipid modification and mitochondria
Abstract
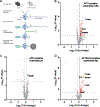
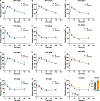
Acyl-protein thioesterases 1 and 2 (APT1 and APT2) reverse S-acylation, a potential regulator of systemic glucose metabolism in mammals. Palmitoylation proteomics in liver-specific knockout mice shows that APT1 predominates over APT2, primarily depalmitoylating mitochondrial proteins, including proteins linked to glutamine metabolism. miniTurbo-facilitated determination of the protein-protein proximity network of APT1 and APT2 in HepG2 cells reveals APT proximity networks encompassing mitochondrial proteins including the major translocases Tomm20 and Timm44. APT1 also interacts with Slc1a5 (ASCT2), the only glutamine transporter known to localize to mitochondria. High-fat-diet-fed male mice with dual (but not single) hepatic deletion of APT1 and APT2 have insulin resistance, fasting hyperglycemia, increased glutamine-driven gluconeogenesis, and decreased liver mass. These data suggest that APT1 and APT2 regulation of hepatic glucose metabolism and insulin signaling is functionally redundant. Identification of substrates and protein-protein proximity networks for APT1 and APT2 establishes a framework for defining mechanisms underlying metabolic disease.
Keywords: CP: Metabolism; acyl-protein thioesterase 1; acyl-protein thioesterase 2; gluconeogenesis; glutamine; insulin; liver; palmitoylation; proximity labeling.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




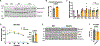
References
-
- Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, and Cusi K (2003). A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 52, 2461–2474. 10.2337/diabetes.52.10.2461. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

