Efficacy and Safety of Teprotumumab in Patients With Thyroid Eye Disease of Long Duration and Low Disease Activity
- PMID: 37925673
- PMCID: PMC10735297
- DOI: 10.1210/clinem/dgad637
Efficacy and Safety of Teprotumumab in Patients With Thyroid Eye Disease of Long Duration and Low Disease Activity
Abstract
Context: Early inflammatory thyroid eye disease (TED) can lead to symptomatic chronic disease, including disabling proptosis. Teprotumumab, an insulin-like growth factor-1 receptor (IGF-1R) inhibitor, previously demonstrated efficacy in acute, high-inflammation TED trials.
Objective: We present data from the first placebo-controlled trial with teprotumumab in chronic/low disease activity TED.
Methods: This randomized double-masked, placebo-controlled trial, conducted at 11 US centers, enrolled adult participants with TED duration of 2 to 10 years, Clinical Activity Score (CAS) ≤ 1 or no additional inflammation or progression in proptosis/diplopia for ≥1 year, proptosis ≥3 mm from before TED and/or from normal, euthyroid/mildly hypo/hyperthyroid, no prior teprotumumab, and no steroids within 3 weeks of baseline. Patients received (2:1) intravenous teprotumumab or placebo once every 3 weeks (total 8 infusions). The primary endpoint was proptosis (mm) improvement at Week 24. Adverse events (AEs) were assessed.
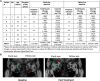
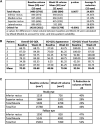
Results: A total of 62 (42 teprotumumab and 20 placebo) patients were randomized. At Week 24, least squares mean (SE) proptosis improvement was greater with teprotumumab (-2.41 [0.228]) than with placebo (-0.92 [0.323]), difference -1.48 (95% CI -2.28, -0.69; P = .0004). Proportions of patients with AEs were similar between groups. Hyperglycemia was reported in 6 (15%) vs 2 (10%) and hearing impairment in 9 (22%) vs 2 (10%) with teprotumumab and placebo, respectively. AEs led to discontinuation in 1 teprotumumab (left ear conductive hearing loss with congenital anomaly) and 1 placebo patient (infusion-related). There were no deaths.
Conclusion: Teprotumumab significantly improved proptosis vs placebo in longstanding/low inflammation TED, demonstrating efficacy regardless of disease duration/activity. The safety profile was comparable to that previously reported.
Keywords: Graves disease; chronic; inactive; teprotumumab; thyroid eye disease.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


Comment in
-
Teprotumumab for Inactive Thyroid Eye Disease? The Jury Is Still Out.J Clin Endocrinol Metab. 2024 Aug 13;109(9):e1802-e1803. doi: 10.1210/clinem/dgae052. J Clin Endocrinol Metab. 2024. PMID: 38279938 No abstract available.
References
-
- Wang Y, Padnick-Silver L, Francis-Sedlak M, Holt RJ, Foley C, Douglas RS. Inflammatory and noninflammatory thyroid eye disease: comparison of disease signs, symptoms, and quality of life in patients in the United States. Endocr Pract. 2022;28(9):842‐846. - PubMed

