Association Between Urinary Biomarkers and CKD in Extremely Low Gestational Age Neonates
- PMID: 37926336
- PMCID: PMC10960703
- DOI: 10.1053/j.ajkd.2023.09.008
Association Between Urinary Biomarkers and CKD in Extremely Low Gestational Age Neonates
Abstract
Rationale & objective: Children born before 28 weeks' gestation are at increased risk of chronic kidney disease (CKD). Urine biomarkers may shed light on mechanistic pathways and improve the ability to forecast CKD. We evaluated whether urinary biomarkers in neonates of low gestational age (GA) are associated with a reduced estimated glomerular filtration rate (eGFR) over time.
Study design: A cohort study of neonates with an exploratory case-control study of a subset of the cohort.
Setting & participants: 327 neonates born at 24-27 weeks' gestation with 2-year eGFR data from the PENUT (Preterm Erythropoietin Neuroprotection Trial) and the REPaIReD (Recombinant Erythropoietin for Prevention of Infant Renal Disease) study.
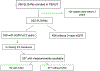
Exposures: 11 urinary biomarkers measured at 27, 30, and 34 weeks' postmenstrual age for the primary cohort study and 10 additional biomarkers for the exploratory case-control study.
Outcomes: eGFR<90mL/min/1.73m2 at 2 years corrected for GA.
Analytical approach: Linear mixed models to assess differences in biomarker values between neonates in whom CKD did and did not develop, accounting for multiple comparisons using Bonferroni-Holm correction in the cohort study only. Cohort analyses were adjusted for sex, GA, and body mass index. Cases were matched to controls on these variables in the case-control study.
Results: After adjusting for weeks of GA, urinary levels of α-glutathione-S-transferase (log difference, 0.27; 95% CI, 0.12-0.43), albumin (log difference, 0.13; 95% CI, 0.02-0.25), and cystatin C (log difference, 0.19; 95% CI, 0.04-0.34) were higher in those in whom CKD developed than in those in whom it did not. Urinary albumin and cystatin C levels did not remain significantly different after Bonferroni-Holm correction. In the exploratory case-control analysis, there were no differences in any biomarkers between cases and controls.
Limitations: Early deaths and a high number of subjects without eGFR at 2 years corrected for GA.
Conclusions: Measurement of urinary biomarkers may assist in monitoring neonates who are at risk for CKD. Additional studies are needed to confirm these findings.
Funding: Grants from government (National Institutes of Health).
Trial registration: Registered at ClinicalTrials.gov with study number NCT01378273.
Plain-language summary: Approximately 15 million neonates worldwide are born prematurely, and 2 million are born before 28 weeks' gestation. Many of these children go on to experience chronic kidney disease. Urine biomarkers may allow for early recognition of those at risk for the development of kidney disease. In this study of more than 300 children born before 28 weeks' gestational age, we found higher mean urinary levels of α-glutathione-S-transferase at 27, 30, and 34 weeks in children whose estimated glomerular filtration rate was<90mL/min/1.73m2 at 2 years compared with children whose estimated glomerular filtration rate was>90mL/min/1.73m2 at 2 years. Measurement of urinary biomarkers may assist in monitoring neonates who are at risk for chronic kidney disease. Additional studies are needed to confirm our findings.
Keywords: extreme prematurity, urinary biomarkers, chronic kidney disease.
Copyright © 2023 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest relevant to this article to disclose.
For full disclosure, we provide here an additional list of other author’s commitments and funding sources that are not directly related to this study. David J Askenazi is a consultant for Baxter, Nuwellis, Bioporto, Seastar and Abbott. His institution has received grant funding for education and research that is not related to this project from NIH, Baxter, Nuwellis, Medtronic, and Seastar, and Leadiant.. He has financial interests in patent/innovations in the area of kidney support therapies and urine collection devices. He is the Founder and Chief Scientific Officer for Zorro-Flow Inc.
Funding sources Recombinant Erythropoietin for Protection of Infant Renal Disease (REPaIReD) Study is an NIH NIDDK funded (R01DK103608) ancillary study designed to look at kidney outcome in patients enrolled in the Preterm Erythropoietin Neuroprotection Trial (PENUT Trial) which is an NIH NINDS funded (U01 NS077953, U01 NS077955) trial. The
Figures




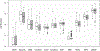
References
-
- Barker DJ. The fetal origins of coronary heart disease. Acta paediatrica. Jul 1997;422:78–82. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

