Upadacitinib as a Rescue Therapy in Acute Severe Ulcerative Colitis: A Case Report and Review of the Literature
- PMID: 37926993
- PMCID: PMC10640891
- DOI: 10.12659/AJCR.940966
Upadacitinib as a Rescue Therapy in Acute Severe Ulcerative Colitis: A Case Report and Review of the Literature
Abstract
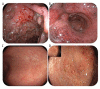
BACKGROUND Ulcerative colitis (UC) is a chronic immune-mediated disease of the colon. The mainstay of treatment to achieve and maintain remission is 5-aminosalicylic acid (5-ASA). At least 20% of patients with UC experience an acute severe ulcerative colitis (ASUC) flare, requiring aggressive early intervention to prevent complications. The first-line treatment of ASUC is intravenous steroids followed by infliximab or cyclosporin in patients for whom steroids fail. Refractory disease failing medical therapy and warranting surgery is common. Lately, Janus kinase (JAK) inhibitors, such as tofacitinib, filgotinib, and upadacitinib, have been licensed for moderate-to-severe UC in adults. Nevertheless, the safety and efficacy of upadacitinib in ASUC has not yet been established. CASE REPORT We report a case of an 18-year-old woman with 4-year history of severe UC. Both infliximab and adalimumab treatments failed, despite the concurrent use of azathioprine, and she was reliant on steroids. Moreover, tofacitinib failed after 1 year of therapy. She was admitted as a case of ASUC. Flexible sigmoidoscopy confirmed severe pancolitis. Finally, she was treated effectively with oral upadacitinib 45 mg given once daily. She went into full clinical, biochemical, and steroid-free remission in 60 days and endoscopic remission at 180 days. CONCLUSIONS This case report features the potential safety and efficacy of upadacitinib in adults with ASUC. Larger trials are required to confirm the efficacy and safety in patients admitted with ASUC.
Conflict of interest statement
Figures
References
-
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5(12):1424–29. - PubMed
-
- Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American college of gastroenterology, practice parameters committee [published erratum appears in Am J Gastroenterol. 2010;105(3):500] Am J Gastroenterol. 2010;105(3):501–23. - PubMed
-
- Hindryckx P, Jairath V, D’Haens G. Acute severe ulcerative colitis: From pathophysiology to clinical management. Nat Rev Gastroenterol Hepatol. 2016;13(11):654–64. - PubMed
-
- Steenholdt C, Ovesen PD, Brynskov J, Seidelin JB. Tofacitinib for acute severe ulcerative colitis: A systematic review. J Crohns Colitis. 2023;17(8):1354–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

