Solvothermal synthesis of cobalt PCP pincer complexes from [Co2(CO)8]
- PMID: 37927400
- PMCID: PMC10620272
- DOI: 10.1007/s00706-023-03123-x
Solvothermal synthesis of cobalt PCP pincer complexes from [Co2(CO)8]
Abstract
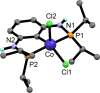
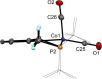
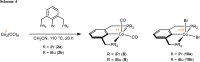
Treatment of [Co2(CO)8] with the ipso-substituted P(C-X)PY ligands (X = Br, Cl; R = iPr, tBu) bearing Y = NH and CH2 linkers under solvothermal conditions affords the five-coordinate Co(I) and Co(III) complexes [CoI(PCPY-R)(CO)2] and [CoIII(PCPY-R)X2]. The later are paramagnetic exhibiting a solution magnetic moment in the range of 3.0-3.3 μB which is consistent with a d6 intermediate spin system corresponding to two unpaired electrons. In the case of P(C-X)PY ligands (X = Br, Cl; R = tBu; Y = NH) the formation of the square planar Co(II) complex [Co(PCPNH-tBu)X] was favored. This complex gives rise to a magnetic moment of 1.8 μB being consistent with a d7 low spin system corresponding to one unpaired electron. All complexes are characterized by means of spectroscopic techniques (NMR, IR), HR-MS. Representative complexes were also characterized by X-ray crystallography.
Supplementary information: The online version contains supplementary material available at 10.1007/s00706-023-03123-x.
Keywords: Cobalt; Oxidative addition; Pincer complexes; Transmetalation.
© The Author(s) 2023.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
