Atypical development of causal inference in autism inferred through a neurocomputational model
- PMID: 37927544
- PMCID: PMC10620690
- DOI: 10.3389/fncom.2023.1258590
Atypical development of causal inference in autism inferred through a neurocomputational model
Abstract
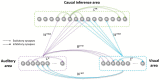
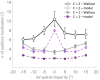
In everyday life, the brain processes a multitude of stimuli from the surrounding environment, requiring the integration of information from different sensory modalities to form a coherent perception. This process, known as multisensory integration, enhances the brain's response to redundant congruent sensory cues. However, it is equally important for the brain to segregate sensory inputs from distinct events, to interact with and correctly perceive the multisensory environment. This problem the brain must face, known as the causal inference problem, is strictly related to multisensory integration. It is widely recognized that the ability to integrate information from different senses emerges during the developmental period, as a function of our experience with multisensory stimuli. Consequently, multisensory integrative abilities are altered in individuals who have atypical experiences with cross-modal cues, such as those on the autistic spectrum. However, no research has been conducted on the developmental trajectories of causal inference and its relationship with experience thus far. Here, we used a neuro-computational model to simulate and investigate the development of causal inference in both typically developing children and those in the autistic spectrum. Our results indicate that higher exposure to cross-modal cues accelerates the acquisition of causal inference abilities, and a minimum level of experience with multisensory stimuli is required to develop fully mature behavior. We then simulated the altered developmental trajectory of causal inference in individuals with autism by assuming reduced multisensory experience during training. The results suggest that causal inference reaches complete maturity much later in these individuals compared to neurotypical individuals. Furthermore, we discuss the underlying neural mechanisms and network architecture involved in these processes, highlighting that the development of causal inference follows the evolution of the mechanisms subserving multisensory integration. Overall, this study provides a computational framework, unifying causal inference and multisensory integration, which allows us to suggest neural mechanisms and provide testable predictions about the development of such abilities in typically developed and autistic children.
Keywords: autism spectrum disorder; causal inference; multisensory integration; multisensory training; neural network; spatial sensory processing; ventriloquism effect.
Copyright © 2023 Monti, Molholm and Cuppini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

