Overcoming barriers: a review on innovations in drug delivery to the middle and inner ear
- PMID: 37927600
- PMCID: PMC10620978
- DOI: 10.3389/fphar.2023.1207141
Overcoming barriers: a review on innovations in drug delivery to the middle and inner ear
Abstract
Despite significant advances in the development of therapeutics for hearing loss, drug delivery to the middle and inner ear remains a challenge. As conventional oral or intravascular administration are ineffective due to poor bioavailability and impermeability of the blood-labyrinth-barrier, localized delivery is becoming a preferable approach for certain drugs. Even then, localized delivery to the ear precludes continual drug delivery due to the invasive and potentially traumatic procedures required to access the middle and inner ear. To address this, the preclinical development of controlled release therapeutics and drug delivery devices have greatly advanced, with some now showing promise clinically. This review will discuss the existing challenges in drug development for treating the most prevalent and damaging hearing disorders, in particular otitis media, perforation of the tympanic membrane, cholesteatoma and sensorineural hearing loss. We will then address novel developments in drug delivery that address these including novel controlled release therapeutics such as hydrogel and nanotechnology and finally, novel device delivery approaches such as microfluidic systems and cochlear prosthesis-mediated delivery. The aim of this review is to investigate how drugs can reach the middle and inner ear more efficiently and how recent innovations could be applied in aiding drug delivery in certain pathologic contexts.
Keywords: biomaterials; drug delivery; hydrogel; inner ear; microdevices; middle ear; nanoparticle.
Copyright © 2023 Delaney, Liew, Lye, Atlas and Wong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
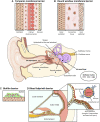
Figures

References
-
- Amable P. R., Carias R. B. V., Teixeira M. V. T., da Cruz Pacheco Í., Corrêa do Amaral R. J. F., Granjeiro J. M., et al. (2013). Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 4 (3), 67–13. 10.1186/scrt218 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

