A high-throughput 3D X-ray histology facility for biomedical research and preclinical applications
- PMID: 37928208
- PMCID: PMC10620852
- DOI: 10.12688/wellcomeopenres.19666.2
A high-throughput 3D X-ray histology facility for biomedical research and preclinical applications
Abstract
Background: The University of Southampton, in collaboration with the University Hospital Southampton (UHS) NHS Foundation Trust and industrial partners, has been at the forefront of developing three-dimensional (3D) imaging workflows using X-ray microfocus computed tomography (μCT) -based technology. This article presents the outcomes of these endeavours and highlights the distinctive characteristics of a μCT facility tailored explicitly for 3D X-ray Histology, with a primary focus on applications in biomedical research and preclinical and clinical studies.
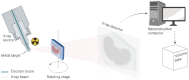
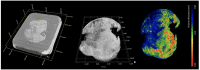
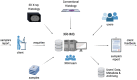
Methods: The UHS houses a unique 3D X-ray Histology (XRH) facility, offering a range of services to national and international clients. The facility employs specialised μCT equipment explicitly designed for histology applications, allowing whole-block XRH imaging of formalin-fixed and paraffin-embedded tissue specimens. It also enables correlative imaging by combining μCT imaging with other microscopy techniques, such as immunohistochemistry (IHC) and serial block-face scanning electron microscopy, as well as data visualisation, image quantification, and bespoke analysis.
Results: Over the past seven years, the XRH facility has successfully completed over 120 projects in collaboration with researchers from 60 affiliations, resulting in numerous published manuscripts and conference proceedings. The facility has streamlined the μCT imaging process, improving productivity and enabling efficient acquisition of 3D datasets.
Discussion & conclusions: The 3D X-ray Histology (XRH) facility at UHS is a pioneering platform in the field of histology and biomedical imaging. To the best of our knowledge, it stands out as the world's first dedicated XRH facility, encompassing every aspect of the imaging process, from user support to data generation, analysis, training, archiving, and metadata generation. This article serves as a comprehensive guide for establishing similar XRH facilities, covering key aspects of facility setup and operation. Researchers and institutions interested in developing state-of-the-art histology and imaging facilities can utilise this resource to explore new frontiers in their research and discoveries.
Keywords: 3D X-ray Histology; 3D histology; Histology; XRH; microCT; virtual histology; μ-CT; μCT.
Copyright: © 2023 Katsamenis OL et al.
Conflict of interest statement
No competing interests were disclosed.
Figures








References
-
- Schneider P, Katsamenis OL, Basford PJ, et al. : Foundations for routine 3D X-ray histology (XRH) in Southampton.In: 8 th annual Tomography for Scientific Advancement (ToScA) Global.Online Edition, Tomography for Scientific Advancement (ToScA), 2020.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

