S100a16 Deficiency Prevents Alcohol-induced Fatty Liver Injury via Inducing MANF Expression in Mice
- PMID: 37928262
- PMCID: PMC10620815
- DOI: 10.7150/ijbs.84472
S100a16 Deficiency Prevents Alcohol-induced Fatty Liver Injury via Inducing MANF Expression in Mice
Abstract
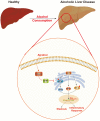
Alcoholic liver disease (ALD) encompasses conditions ranging from simple steatosis to cirrhosis and even liver cancer. It has gained significant global attention in recent years. Despite this, effective pharmacological treatments for ALD remain elusive, and the core mechanisms underlying the disease are not yet fully comprehended. S100A16, a newly identified calcium-binding protein, is linked to lipid metabolism. Our research has discovered elevated levels of the S100A16 protein in both serum and liver tissue of ALD patients. A similar surge in hepatic S100A16 expression was noted in a Gao-binge alcohol feeding mouse model. S100a16 knockdown alleviated ethanol-induced liver injury, steatosis and inflammation. Conversely, S100a16 transgenic mice showed aggravating phenomenon. Mechanistically, we identify mesencephalic astrocyte-derived neurotrophic factor (MANF) as a regulated entity downstream of S100a16 deletion. MANF inhibited ER-stress signal transduction induced by alcohol stimulation. Meanwhile, MANF silencing suppressed the inhibition effect of S100a16 knockout on ethanol-induced lipid droplets accumulation in primary hepatocytes. Our data suggested that S100a16 deletion protects mice against alcoholic liver lipid accumulation and inflammation dependent on upregulating MANF and inhibiting ER stress. This offers a potential therapeutic avenue for ALD treatment.
Keywords: Alcoholic liver disease; ER stress.; MANF; S100A16; hepatic steatosis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

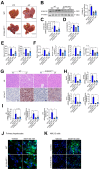
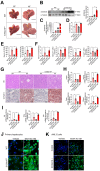
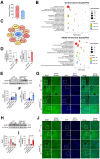
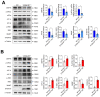

References
-
- Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58(3):593–608. - PubMed
-
- Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L. et al. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69(3):718–735. - PubMed
-
- WHO. Global status report on alcohol and health 2014. 2014.
-
- O'Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology. 2010;51(1):307–28. - PubMed
-
- Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12(4):231–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

