Heterologous prime-boost H1N1 vaccination exacerbates disease following challenge with a mismatched H1N2 influenza virus in the swine model
- PMID: 37928521
- PMCID: PMC10623127
- DOI: 10.3389/fimmu.2023.1253626
Heterologous prime-boost H1N1 vaccination exacerbates disease following challenge with a mismatched H1N2 influenza virus in the swine model
Abstract
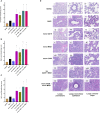
Influenza A viruses (IAVs) pose a significant threat to both human and animal health. Developing IAV vaccine strategies able to elicit broad heterologous protection against antigenically diverse IAV strains is pivotal in effectively controlling the disease. The goal of this study was to examine the immunogenicity and protective efficacy of diverse H1N1 influenza vaccine strategies including monovalent, bivalent, and heterologous prime-boost vaccination regimens, against a mismatched H1N2 swine influenza virus. Five groups were homologous prime-boost vaccinated with either an oil-adjuvanted whole-inactivated virus (WIV) monovalent A/swine/Georgia/27480/2019 (GA19) H1N2 vaccine, a WIV monovalent A/sw/Minnesota/A02636116/2021 (MN21) H1N1 vaccine, a WIV monovalent A/California/07/2009 (CA09) H1N1, a WIV bivalent vaccine composed of CA09 and MN21, or adjuvant only (mock-vaccinated group). A sixth group was prime-vaccinated with CA09 WIV and boosted with MN21 WIV (heterologous prime-boost group). Four weeks post-boost pigs were intranasally and intratracheally challenged with A/swine/Georgia/27480/2019, an H1N2 swine IAV field isolate. Vaccine-induced protection was evaluated based on five critical parameters: (i) hemagglutination inhibiting (HAI) antibody responses, (ii) clinical scores, (iii) virus titers in nasal swabs and respiratory tissue homogenates, (iv) BALf cytology, and (v) pulmonary pathology. While all vaccination regimens induced seroprotective titers against homologous viruses, heterologous prime-boost vaccination failed to enhance HAI responses against the homologous vaccine strains compared to monovalent vaccine regimens and did not expand the scope of cross-reactive antibody responses against antigenically distinct swine and human IAVs. Mismatched vaccination regimens not only failed to confer clinical and virological protection post-challenge but also exacerbated disease and pathology. In particular, heterologous-boosted pigs showed prolonged clinical disease and increased pulmonary pathology compared to mock-vaccinated pigs. Our results demonstrated that H1-specific heterologous prime-boost vaccination, rather than enhancing cross-protection, worsened the clinical outcome and pathology after challenge with the antigenically distant A/swine/Georgia/27480/2019 strain.
Keywords: hemagglutinin; heterologous prime-boost; influenza A virus; swine; vaccine.
Copyright © 2023 Pliasas, Neasham, Naskou, Neto, Strate, North, Pedroza, Chastain, Padykula, Tompkins and Kyriakis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Piroth L, Cottenet J, Mariet A-S, Bonniaud P, Blot M, Tubert-Bitter P, et al. Comparison of the characteristics, morbidity, and mortality of Covid-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir Med (2021) 9(3):251–9. doi: 10.1016/S2213-2600(20)30527-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

