Albumins represent highly cross-reactive animal allergens
- PMID: 37928538
- PMCID: PMC10623431
- DOI: 10.3389/fimmu.2023.1241518
Albumins represent highly cross-reactive animal allergens
Abstract
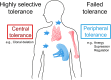
Albumins from animals are highly cross-reactive allergens for patients suffering from immunoglobulin E (IgE)-mediated allergy. Approximately 20-30% of cat and dog allergic patients show IgE reactivity and mount IgE-mediated allergic reactions to cat and dog albumin. It is astonishing that allergic patients can develop specific IgE responses against animal albumins because these proteins exhibit a more than 70% sequence identity to human serum albumin (HSA) which is the most abundant protein in the blood of the human body. The sequence identity of cat albumin (Fel d 2) and dog albumin (Can f 3) and HSA are 82% and 80%, respectively. Given the high degree of sequence identity between the latter two allergens and HSA one would expect that immunological tolerance would prohibit IgE sensitization to Fel d 2 and Can f 3. Here we discuss two possibilities for how IgE sensitization to Fel d 2 and Can f 3 may develop. One possibility is the failed development of immune tolerance in albumin-allergic patients whereas the other possibility is highly selective immune tolerance to HSA but not to Fel d 2 and Can f 3. If the first assumption is correct it should be possible to detect HSA-specific T cell responses and HSA-containing immune complexes in sensitized patients. In the latter scenario few differences in the sequences of Fel d 2 and Can f 3 as compared to HSA would be responsible for the development of selective T cell and B cell responses towards Fel d 2 as well as Can f 3. However, the immunological mechanisms of albumin sensitization have not yet been investigated in detail although this will be important for the development of allergen-specific prevention and allergen-specific immunotherapy (AIT) strategies for allergy to albumin.
Keywords: albumin; allergen; allergy; antibody; cross-reactivity; epitope; immunoglobulin E (IgE); tolerance.
Copyright © 2023 Liu, Trifonova, Tulaeva, Riabova, Karsonova, Kozlov, Elisyutina, Khaitov, Focke-Tejkl, Chen, Karaulov and Valenta.
Conflict of interest statement
Author T-HC was employed by the company Worg Pharmaceuticals. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

