NK cell subsets and dysfunction during viral infection: a new avenue for therapeutics?
- PMID: 37928543
- PMCID: PMC10620977
- DOI: 10.3389/fimmu.2023.1267774
NK cell subsets and dysfunction during viral infection: a new avenue for therapeutics?
Abstract
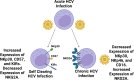
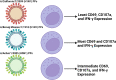
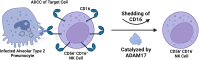
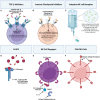
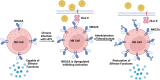
In the setting of viral challenge, natural killer (NK) cells play an important role as an early immune responder against infection. During this response, significant changes in the NK cell population occur, particularly in terms of their frequency, location, and subtype prevalence. In this review, changes in the NK cell repertoire associated with several pathogenic viral infections are summarized, with a particular focus placed on changes that contribute to NK cell dysregulation in these settings. This dysregulation, in turn, can contribute to host pathology either by causing NK cells to be hyperresponsive or hyporesponsive. Hyperresponsive NK cells mediate significant host cell death and contribute to generating a hyperinflammatory environment. Hyporesponsive NK cell populations shift toward exhaustion and often fail to limit viral pathogenesis, possibly enabling viral persistence. Several emerging therapeutic approaches aimed at addressing NK cell dysregulation have arisen in the last three decades in the setting of cancer and may prove to hold promise in treating viral diseases. However, the application of such therapeutics to treat viral infections remains critically underexplored. This review briefly explores several therapeutic approaches, including the administration of TGF-β inhibitors, immune checkpoint inhibitors, adoptive NK cell therapies, CAR NK cells, and NK cell engagers among other therapeutics.
Keywords: CMV; HIV; NK cell dysfunction; NK cells; SARS-CoV-2; hepatitis C; immunotherapy; influenza.
Copyright © 2023 Bjorgen, Dick, Cromarty, Hart and Rhein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

