Ferroptosis as an emerging target in rheumatoid arthritis
- PMID: 37928554
- PMCID: PMC10620966
- DOI: 10.3389/fimmu.2023.1260839
Ferroptosis as an emerging target in rheumatoid arthritis
Abstract
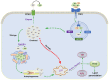
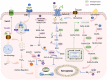
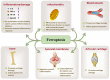
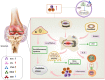
Rheumatoid arthritis (RA) is an autoimmune disease of unknown etiology. Due to the rise in the incidence rate of RA and the limitations of existing therapies, the search for new treatment strategies for RA has become a global focus. Ferroptosis is a novel programmed cell death characterized by iron-dependent lipid peroxidation, with distinct differences from apoptosis, autophagy, and necrosis. Under the conditions of iron accumulation and the glutathione peroxidase 4 (GPX4) activity loss, the lethal accumulation of lipid peroxide is the direct cause of ferroptosis. Ferroptosis mediates inflammation, oxidative stress, and lipid oxidative damage processes, and also participates in the occurrence and pathological progression of inflammatory joint diseases including RA. This review provides insight into the role and mechanism of ferroptosis in RA and discusses the potential and challenges of ferroptosis as a new therapeutic strategy for RA, with an effort to provide new targets for RA prevention and treatment.
Keywords: emerging target; ferroptosis; iron metabolism; lipid peroxidation; rheumatoid arthritis.
Copyright © 2023 Zhao, Tang, Wang, Zhao and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Osipova D, Janssen R, Martens HA. Rheumatoid arthritis: more than a joint disease. Ned Tijdschr Geneeskd (2020) 164:D4166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

