Oral bemnifosbuvir (AT-527) vs placebo in patients with mild-to-moderate COVID-19 in an outpatient setting (MORNINGSKY)
- PMID: 37928891
- PMCID: PMC10621114
- DOI: 10.2217/fvl-2023-0115
Oral bemnifosbuvir (AT-527) vs placebo in patients with mild-to-moderate COVID-19 in an outpatient setting (MORNINGSKY)
Abstract
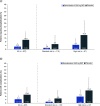
Aim: This phase III study assessed the efficacy/safety/antiviral activity/pharmacokinetics of bemnifosbuvir, a novel, oral nucleotide analog to treat COVID-19. Patients & methods: Outpatient adults/adolescents with mild-to-moderate COVID-19 were randomized 2:1 to bemnifosbuvir/placebo. Time to symptom alleviation/improvement (primary outcome), risk of hospitalization/death, viral load and safety were evaluated. Results: Although the study was discontinued prematurely and did not meet its primary end point, bemnifosbuvir treatment resulted in fewer hospitalizations (71% relative risk reduction), COVID-19-related medically attended hospital visits, and COVID-19-related complications compared with placebo. No reduction in viral load was observed. The proportion of patients with adverse events was similar; no deaths occurred. Conclusion: Bemnifosbuvir showed hospitalization reduction in patients with variable disease progression risk and was well tolerated. Clinical Trial Registration: NCT04889040 (ClinicalTrials.gov).
Keywords: AT-527; COVID-19; SARS-CoV-2; bemnifosbuvir; oral; outpatient.
© 2023 The Authors.
Conflict of interest statement
Competing interests disclosure A Horga, K Pietropaolo, L Ishak and B Belanger are employees of Atea. R Saenz is an employee and owns stock for Genentech (a member of the Roche group). A Simón-Campos is a speaker and advisory board member for Pfizer, AstraZeneca, Roche and Regeneron. WJ Stubbings, N Collinson, C Granier, AC Hurt and S Wildum are employees and shareholders of F. Hoffman-La-Roche Ltd. B Zrinscak is an employee of F. Hoffman-La-Roche Ltd. K Lin is a former employee of Atea. X-J Zhou and J Hammond are employees and shareholders of Atea. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures


References
-
- Salian VS, Wright JA, Vedell PT et al. COVID-19 transmission, current treatment, and future therapeutic strategies. Mol Pharm. 18(3), 754–771 (2021). - PubMed
-
- Di Fusco M, Shea KM, Lin J et al. Health outcomes and economic burden of hospitalized COVID-19 patients in the United States. J. Med. Econ. 24(1), 308–317 (2021). - PubMed
-
- Iuliano AD, Brunkard JM, Boehmer TK et al. Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb. Mortal. Wkly Rep. 71(4), 146–152 (2022). - PMC - PubMed
-
- Mathieu E, Ritchie H, Ortiz-Ospina E et al. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 5(7), 947–953 (2021). - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
