Assay Development for Metal-Dependent Enzymes-Influence of Reaction Buffers on Activities and Kinetic Characteristics
- PMID: 37929113
- PMCID: PMC10620931
- DOI: 10.1021/acsomega.3c02835
Assay Development for Metal-Dependent Enzymes-Influence of Reaction Buffers on Activities and Kinetic Characteristics
Abstract
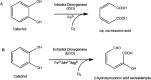
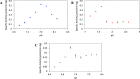
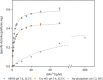
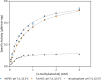
Buffers are often thought of as innocuous components of a reaction, with the sole task of maintaining the pH of a system. However, studies had shown that this is not always the case. Common buffers used in biochemical research, such as Tris (hydroxymethyl) aminomethane hydrochloride (Tris-HCl), can chelate metal ions and may thus affect the activity of metalloenzymes, which are enzymes that require metal ions for enhanced catalysis. To determine whether enzyme activity is influenced by buffer identity, the activity of three enzymes (BLC23O, Ro1,2-CTD, and trypsin) was comparatively characterized in N-2- hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES), Tris-HCl, and sodium phosphate buffer. The pH and temperature optima of BLC23O, a Mn2+-dependent dioxygenase, were first identified, and then the metal ion dissociation constant (Kd) was determined in the three buffer systems. It was observed that BLC23O exhibited different Kd values depending on the buffer, with the lowest (1.49 ± 0.05 μM) recorded in HEPES under the optimal set of conditions (pH 7.6 and 32.5 °C). Likewise, the kinetic parameters obtained varied depending on the buffer, with HEPES (pH 7.6) yielding overall the greatest catalytic efficiency and turnover number (kcat = 0.45 ± 0.01 s-1; kcat/Km = 0.84 ± 0.02 mM-1 s-1). To corroborate findings, the characterization of Fe3+-dependent Ro1,2-CTD was performed, resulting in different kinetic constants depending on the buffer (Km (HEPES, Tris-HCl, and Na-phosphate) = 1.80, 6.93, and 3.64 μM; kcat(HEPES, Tris-HCl, and Na-phosphate) = 0.64, 1.14, and 1.01 s-1; kcat/Km(HEPES, Tris-HCl, and Na-phosphate)= 0.36, 0.17, and 0.28 μM-1 s-1). In order to determine whether buffer identity influenced the enzymatic activity of nonmetalloenzymes alike, the characterization of trypsin was also carried out. Contrary to the previous results, trypsin yielded comparable kinetic parameters independent of the buffer (Km (HEPES, Tris-HCl, and Na-Phosphate) = 3.14, 3.07, and 2.91 mM; kcat(HEPES, Tris-HCl, and Na-phosphate) = 1.51, 1.47, and 1.53 s-1; kcat/Km (HEPES, Tris-HCl, and Na-phosphate) = 0.48, 0.48, and 0.52 mM-1 s-1). These results showed that the activity of tested metalloenzymes was impacted by different buffers. While selected buffers did not influence the tested nonmetalloenzyme activity, other research had shown impacts of buffers on other enzyme activities. As a result, we suggest that buffer selection be optimized for any new enzymes such that the results from one lab to another can be accurately compared.
Crown © 2023. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Ferreira C. M.; Pinto I. S.; Soares E. V.; Soares H. M. (Un)Suitability of the use of pH buffers in biological, biochemical and environmental studies and their interaction with metal ions – a review. RSC Adv. 2015, 5, 30989–31003. 10.1039/C4RA15453C. - DOI
-
- Fischer B. E.; Haring U. K.; Tribolet R.; Sigel H. Metal Ion/Buffer Interactions. Stability of Binary and Ternary Complexes Containing 2-Amino-2(hydroxymethyl)-1,3-propanediol (Tris) and Adenosine 5′-Triphosphate (ATP). Eur. J. Biochem. 1979, 94, 523–530. 10.1111/j.1432-1033.1979.tb12921.x. - DOI - PubMed
-
- Blanchard J. S.Buffers for enzymes. In Methods in Enzymology; Academic Press: Cambridge, MA, 1984; 104, pp. 404–414. - PubMed
-
- Guzik U.; Hupert-Kocurek K.; Wojcieszysk D. Intradiol Dioxygenases — The Key Enzymes in Xenobiotics Degradation. Biodegradation of Hazardous and Special Products 2013, 129.10.5772/56205. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

