Antimycobacterial Precatorin A Flavonoid Displays Antibiofilm Activity against Mycobacterium bovis BCG
- PMID: 37929145
- PMCID: PMC10621015
- DOI: 10.1021/acsomega.3c05703
Antimycobacterial Precatorin A Flavonoid Displays Antibiofilm Activity against Mycobacterium bovis BCG
Abstract
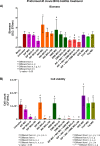
The aim of this study was to evaluate the potential antibiofilm activity of Rhynchosia precatoria (R. precatoria) compounds over Mycobacterium bovis BCG (M. bovis BCG) as a model for Mycobacterium tuberculosis (Mtb). We evaluated the antibiofilm activity as the ability to both inhibit biofilm formation and disrupt preformed biofilms (bactericidal) of R. precatoria compounds, which have been previously described as being antimycobacterials against Mtb. M. bovis BCG developed air-liquid interface biofilms with surface attachment ability and drug tolerance. Of the R. precatoria extracts and compounds that were tested, precatorin A (PreA) displayed the best biofilm inhibitory activity, as evaluated by biofilm biomass quantification, viable cell count, and confocal and atomic force microscopy procedures. Furthermore, its combination with isoniazid at subinhibitory concentrations inhibited M. bovis BCG biofilm formation. Nonetheless, neither PreA nor the extract showed bactericidal effects. PreA is the R. precatoria compound responsible for biofilm inhibitory activity against M. bovis BCG.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
New Isoflavonoids from the extract of Rhynchosia precatoria (Humb. & Bonpl. ex Willd.) DC. and their antimycobacterial activity.J Ethnopharmacol. 2017 Jul 12;206:92-100. doi: 10.1016/j.jep.2017.05.019. Epub 2017 May 12. J Ethnopharmacol. 2017. PMID: 28506901
-
Antimycobacterial activity of medicinal plants used by the Mayo people of Sonora, Mexico.J Ethnopharmacol. 2016 Aug 22;190:106-15. doi: 10.1016/j.jep.2016.05.064. Epub 2016 Jun 1. J Ethnopharmacol. 2016. PMID: 27262564
-
Purified compounds and extracts from Euclea species with antimycobacterial activity against Mycobacterium bovis and fast-growing mycobacteria.Biol Pharm Bull. 2008 Jul;31(7):1429-33. doi: 10.1248/bpb.31.1429. Biol Pharm Bull. 2008. PMID: 18591787
-
Three-dimensional low shear culture of Mycobacterium bovis BCG induces biofilm formation and antimicrobial drug tolerance.NPJ Biofilms Microbiomes. 2021 Feb 1;7(1):12. doi: 10.1038/s41522-021-00186-8. NPJ Biofilms Microbiomes. 2021. PMID: 33526771 Free PMC article.
-
Inhibition of biofilm formation in Mycobacterium smegmatis by Parinari curatellifolia leaf extracts.BMC Complement Altern Med. 2017 May 30;17(1):285. doi: 10.1186/s12906-017-1801-5. BMC Complement Altern Med. 2017. PMID: 28558683 Free PMC article.
Cited by
-
Ajoene: a natural compound with enhanced antimycobacterial and antibiofilm properties mediated by efflux pump modulation and ROS generation against M. Smegmatis.Arch Microbiol. 2024 Nov 2;206(12):453. doi: 10.1007/s00203-024-04189-9. Arch Microbiol. 2024. PMID: 39487375
-
Alleviation of mycobacterial infection by impairing motility and biofilm formation via natural and synthetic molecules.World J Microbiol Biotechnol. 2025 Mar 28;41(4):113. doi: 10.1007/s11274-025-04322-w. World J Microbiol Biotechnol. 2025. PMID: 40148661 Review.
References
-
- World Health Organization . Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021.
LinkOut - more resources
Full Text Sources

