Inflammation and Cholesterol as Predictors of Cardiovascular Events Among 13 970 Contemporary High-Risk Patients With Statin Intolerance
- PMID: 37929602
- PMCID: PMC10752259
- DOI: 10.1161/CIRCULATIONAHA.123.066213
Inflammation and Cholesterol as Predictors of Cardiovascular Events Among 13 970 Contemporary High-Risk Patients With Statin Intolerance
Abstract
Background: Among patients treated with statin therapy to guideline-recommended cholesterol levels, residual inflammatory risk assessed by high-sensitivity C-reactive protein (hsCRP) is at least as strong a predictor of future cardiovascular events as is residual risk assessed by low-density lipoprotein cholesterol (LDLC). Whether these relationships are present among statin-intolerant patients with higher LDLC levels is uncertain but has implications for the choice of preventive therapies, including bempedoic acid, an agent that reduces both LDLC and hsCRP.
Methods: The multinational CLEAR-Outcomes trial (Cholesterol Lowering via Bempedoic Acid, an ACL-Inhibiting Regimen Outcomes Trial) randomly allocated 13 970 statin-intolerant patients to 180 mg of oral bempedoic acid daily or matching placebo and followed them for a 4-component composite of incident myocardial infarction, stroke, coronary revascularization, or cardiovascular death, and for all-cause mortality. Quartiles of increasing baseline hsCRP and LDLC were assessed as predictors of future adverse events after adjustment for traditional risk factors and randomized treatment assignment.
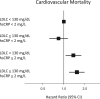
Results: Compared with placebo, bempedoic acid reduced median hsCRP by 21.6% and mean LDLC levels by 21.1% at 6 months. Baseline hsCRP was significantly associated with the primary composite end point of major cardiovascular events (highest versus lowest hsCRP quartile; hazard ratio [HR], 1.43 [95% CI, 1.24-1.65]), cardiovascular mortality (HR, 2.00 [95% CI, 1.53-2.61]), and all-cause mortality (HR, 2.21 [95% CI, 1.79-2.73]). By contrast, the relationship of baseline LDLC quartile (highest versus lowest) to future events was smaller in magnitude for the primary composite cardiovascular end point (HR, 1.19 [95% CI, 1.04-1.37]) and neutral for cardiovascular mortality (HR, 0.90 [95% CI, 0.70-1.17]) and all-cause mortality (HR, 0.95 [95% CI, 0.78-1.16]). Risks were high for those with elevated hsCRP irrespective of LDLC level. Bempedoic acid demonstrated similar efficacy in reducing cardiovascular events across all levels of hsCRP and LDLC.
Conclusions: Among contemporary statin-intolerant patients, inflammation assessed by hsCRP predicted risk for future cardiovascular events and death more strongly than hyperlipidemia assessed by LDLC. Compared with placebo, bempedoic acid had similar efficacy for reducing cardiovascular risk across hsCRP and LDLC strata.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT02993406.
Keywords: C-reactive protein; atherosclerosis; cholesterol; clinical trials as topic; inflammation.
Conflict of interest statement
Figures

Comment in
-
Bempedoic acid reduces the risk of cardiovascular events in statin-intolerant patients.Nat Cardiovasc Res. 2024 Feb;3(2):96. doi: 10.1038/s44161-024-00435-x. Nat Cardiovasc Res. 2024. PMID: 39196197 No abstract available.
References
-
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401 - PubMed
-
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202 - PubMed
-
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914 - PubMed
-
- Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388 - PubMed
-
- Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu X-F, Ireland MA, Lenderink T, et al. ; LoDoCo2 Trial Investigators. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

