Midterm Survival of Low-Risk Patients Treated With Transcatheter Versus Surgical Aortic Valve Replacement: Meta-Analysis of Reconstructed Time-to-Event Data
- PMID: 37929669
- PMCID: PMC10727380
- DOI: 10.1161/JAHA.123.030012
Midterm Survival of Low-Risk Patients Treated With Transcatheter Versus Surgical Aortic Valve Replacement: Meta-Analysis of Reconstructed Time-to-Event Data
Abstract
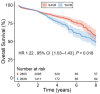
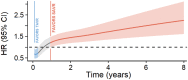
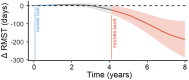
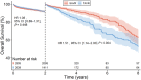
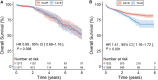
Background We performed a meta-analysis of reconstructed time-to-event data from randomized controlled trials (RCTs) and propensity-score matched (PSM) studies comparing transcatheter versus surgical aortic valve replacement (TAVR versus SAVR) to evaluate midterm outcomes in patients considered low risk for SAVR. Methods and Results Study-level meta-analysis of reconstructed time-to-event data from Kaplan-Meier curves of RCTs and PSM studies published by December 31, 2022 was conducted. Eight studies (3 RCTs, 5 PSM studies) met our eligibility criteria and included 5444 patients; 2639 patients underwent TAVR, and 2805 patients underwent SAVR. TAVR showed a higher risk of all-cause mortality at 8 years of follow-up (hazard ratio [HR] 1.22, [95% CI, 1.03-1.43], P=0.018). Up to 2 years of follow-up, TAVR was not inferior to SAVR (HR, 1.08 [95% CI, 0.89-1.31], P=0.448); however, we observed a statistically significant difference after 2 years with higher mortality with TAVR (HR, 1.51 [95% CI, 1.14-2.00]; P=0.004). This difference was driven by PSM studies; our sensitivity analysis showed a statistically significant difference between TAVR and SAVR when we included only PSM studies (HR, 1.41 [95% CI, 1.16-1.72], P=0.001) but no statistically significant difference when we included only RCTs (HR, 0.89 [95% CI, 0.69-1.16], P=0.398). Conclusions In comparison with TAVR, SAVR appeared to be associated with improved survival beyond 2 years in low-risk patients. However, the survival benefit of SAVR was observed only in PSM studies and not in RCTs. The addition of data from ongoing RCTs as well as longer follow-up in previous RCTs will help to confirm if there is a difference in mid- and long-term survival between TAVR versus SAVR in the low-risk population.
Keywords: cardiac surgical procedures; cardiovascular surgical procedures; heart valve diseases; heart valve prosthesis implantation; meta‐analysis; transcatheter aortic valve replacement.
Figures
Comment in
-
Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients: Puzzle Solved?J Am Heart Assoc. 2023 Nov 7;12(21):e030953. doi: 10.1161/JAHA.123.030953. Epub 2023 Nov 6. J Am Heart Assoc. 2023. PMID: 37929671 Free PMC article. No abstract available.
References
-
- Barili F, Freemantle N, Pilozzi Casado A, Rinaldi M, Folliguet T, Musumeci F, Gerosa G, Parolari A. Mortality in trials on transcatheter aortic valve implantation versus surgical aortic valve replacement: a pooled meta‐analysis of Kaplan‐Meier‐derived individual patient data. Eur J Cardiothorac Surg. 2020;58:221–229. doi: 10.1093/ejcts/ezaa087 - DOI - PubMed
-
- Barili F, Freemantle N, Musumeci F, Martin B, Anselmi A, Rinaldi M, Kaul S, Rodriguez‐Roda J, Di Mauro M, Folliguet T, et al. Five‐year outcomes in trials comparing transcatheter aortic valve implantation versus surgical aortic valve replacement: a pooled meta‐analysis of reconstructed time‐to‐event data. Eur J Cardiothorac Surg. 2022;61:977–987. doi: 10.1093/ejcts/ezab516 - DOI - PubMed
-
- Thourani VH, Suri RM, Gunter RL, Sheng S, O'Brien SM, Ailawadi G, Szeto WY, Dewey TM, Guyton RA, Bavaria JE, et al. Contemporary real‐world outcomes of surgical aortic valve replacement in 141,905 low‐risk, intermediate‐risk, and high‐risk patients. Ann Thorac Surg. 2015;99:55–61. doi: 10.1016/j.athoracsur.2014.06.050 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

