Carriage and within-host diversity of mcr-1.1- harbouring Escherichia coli from pregnant mothers: inter- and intra-mother transmission dynamics of mcr-1.1
- PMID: 37929689
- PMCID: PMC10773534
- DOI: 10.1080/22221751.2023.2278899
Carriage and within-host diversity of mcr-1.1- harbouring Escherichia coli from pregnant mothers: inter- and intra-mother transmission dynamics of mcr-1.1
Abstract
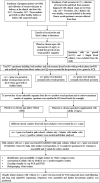
Exchange of antimicrobial resistance genes via mobile genetic elements occur in the gut which can be transferred from mother to neonate during birth. This study is the first to analyse transmissible colistin resistance gene, mcr, in pregnant mothers and neonates. Samples were collected from pregnant mothers (rectal) and septicaemic neonates (rectal and blood) and analysed for the presence of mcr, its transmissibility, genome diversity, and exchange of mcr between isolates within an individual and across different individuals (not necessarily mother-baby pairs). mcr-1.1 was detected in rectal samples of pregnant mothers (n = 10, 0.9%), but not in neonates. All mcr-positive mothers gave birth to healthy neonates from whom rectal specimen were not collected. Hence, the transmission of mcr between these mother-neonate pairs could not be studied. mcr-1.1 was noted only in Escherichia coli (phylogroup A & B1), and carried few resistance and virulence genes. Isolates belonged to diverse sequence types (n = 11) with two novel STs (ST12452, ST12455). mcr-1.1 was borne on conjugative IncHI2 bracketed between ISApl1 on Tn6630, and the plasmids exhibited similarities in sequences across the study isolates. Phylogenetic comparison showed that study isolates were related to mcr-positive isolates of animal origin from Southeast Asian countries. Spread of mcr-1.1 within this study occurred either via similar mcr-positive clones or similar mcr-bearing plasmids in mothers. Though this study could not build evidence for mother-baby transmission but the presence of such genes in the maternal specimen may enhance the chances of transmission to neonates.
Keywords: Colistin-resistant Escherichia coli; Illumina & MinION nanopore sequencing; mcr-1.1-bearing IncHI2; pregnant mother and neonatal gut carriage; transmission dynamics of mcr-1.1.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
