Phosphodiesterase 5 inhibitors (PDE5i) for the treatment of Raynaud's phenomenon
- PMID: 37929840
- PMCID: PMC10626647
- DOI: 10.1002/14651858.CD014089
Phosphodiesterase 5 inhibitors (PDE5i) for the treatment of Raynaud's phenomenon
Abstract
Background: Raynaud's phenomenon is a vasodilatory phenomenon characterised by digital pallor, cyanosis, and pain of the extremities. Primary Raynaud's phenomenon has no underlying disease associated with it, while secondary Raynaud's phenomenon is associated with connective tissue disorders such as systemic sclerosis. Systemic sclerosis causes fibrosis and commonly affects the skin and internal organs such as the gastrointestinal tract, lungs, kidney, and heart. Phosphodiesterase 5 inhibitors (PDE5i) are a class of drugs that increases blood flow to the extremities and may be beneficial in the treatment of Raynaud's phenomenon.
Objectives: To assess the benefits and harms of PDE5i compared to placebo for the treatment of Raynaud's phenomenon.
Search methods: We searched the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, and clinical trial registries up to June 2022. We did not apply any language restrictions. We searched the bibliographies of retrieved articles and contacted key experts in the field for additional and unpublished data.
Selection criteria: Randomised controlled trials (RCTs) comparing PDE5i to placebo in people with primary and secondary Raynaud's phenomenon.
Data collection and analysis: We used standard methodological procedures expected by Cochrane.
Main results: This review included nine RCTs which ranged in duration from four to eight weeks and included a total of 411 participants. The majority had Raynaud's phenomenon secondary to systemic sclerosis. Tadalafil was assessed in four studies, sildenafil in three studies, vardenafil in one study, and a new PDE5 inhibitor known as "PF-00489791" in one study. Three studies were parallel design and six studies were cross-over. The frequency of attacks per week was 24 with placebo and PDE5i reduced the frequency of attacks by an average of three attacks per week (mean difference (MD) -3.07, 95% confidence interval (CI) -5.15 to -1.00; 8 studies; low-certainty evidence). The duration of attacks per day was 55 minutes with placebo and PDE5i reduced the duration of attacks by an average of five minutes (MD -5.31, 95% CI -8.90 to -1.71; 8 studies; low-certainty evidence). Very low-certainty evidence from one study with eight participants showed severity of Raynaud's attacks (assessed on a 10 cm visual analogue scale with lower scores indicating less severity) was 20% lower with a PDE5i (3.7 with placebo compared to 1.6 with treatment; MD -2.1, 95% CI -2.7 to 1.4; very low-certainty evidence). Pain and patient global assessment were assessed on a 10 cm visual analogue scale with lower scores indicating improvement. Low-certainty evidence showed that the use of PDE5i may result in little to no difference compared to placebo in reducing the average pain of Raynaud's attacks (3 to 2.9; MD -0.10, 95% CI -0.78 to 0.57; 4 studies). Global scores were 36% lower with the use of a PDE5i compared to placebo (9.2 to 5.6; MD -3.59, 95% CI -4.45 to -2.73; 1 study, 24 participants; low-certainty evidence). The rate of withdrawals during treatment with PDE5i ranged from 2% to 20% compared with 2% to 4% in the placebo group in five studies. Four studies reported no withdrawals due to adverse events. Seven studies reported no serious adverse events. The rate of serious adverse events reported in two studies ranFged from 2% during treatment to 4% with placebo. The majority of the studies were judged as low or unclear risk of bias for selection, performance, and detection bias. Almost half were judged at high risk of attrition bias and unclear risk for selective reporting bias. We downgraded frequency of attacks, duration of attacks, pain intensity, and patient global assessment for small sample sizes and concerns about inconsistency and graded each as low certainty of evidence. We downgraded severity of attacks to very low certainty due to serious concerns about imprecision and publication bias. We downgraded withdrawals due to adverse events and serious adverse events to moderate certainty of evidence due to a low number of reported events.
Authors' conclusions: Based on low-certainty evidence, PDE5i may reduce the frequency of attacks of Raynaud's phenomenon by a small amount per week, result in a small reduction in the duration of attack, improve patients' global assessment of their disease, and result in little to no difference in pain. PDE5i probably result in little or no difference in serious adverse events but slightly increase the likelihood of withdrawing from treatment due to an adverse event.
Trial registration: ClinicalTrials.gov NCT01117298 NCT01347008 NCT00822354 NCT02050360.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
PT: Independent Contractor/Consultant for Data And Safety Monitoring Board, Reformulary Group, Outcome Measures in Rheumatology (OMERACT).
GAW: University of Ottawa (Employment)
JP, NM, LJM, EG, SEH, PCT, FR: none known
Figures
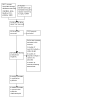

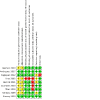
























References
References to studies included in this review
Agarwal 2010 {published and unpublished data}
-
- Agarwal V, Ghosh P, Sharma A, Bhakuni DS, Kumar S, Singh UM, et al. Efficacy of Tadalafil in Raynaud’s phenomenon secondary to systemic sclerosis: a double blind randomized placebo controlled parallel group multicentric study. Arthritis and Rheumatism 2010;62 Suppl 10:2086. [CLINICALTRIALS.GOV: NCT01117298]
Andrigueti 2017 {published data only}
-
- Andrigueti FV, Ebbing PC, Arismendi MI, Kayser C. Evaluation of the effect of Sildenafil on the microvascular blood flow and on the endothelial progenitor cells in patients with early systemic sclerosis: a randomized, double-blind, placebo-controlled study. In: American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting; San Diego (CA). 2013 Oct 25-30. [Abstract 1813]
-
- Andrigueti FV, Ebbing PC, Arismendi MI, Kayser C. Evaluation of the effect of sildenafil on the microvascular blood flow in patients with systemic sclerosis: a randomised, double-blind, placebo-controlled study. Clinical and Experimental Rheumatology 2017;35 Suppl 106:S151-8. - PubMed
Caglayan 2012 {published data only}
-
- Caglayan E, Axmann S, Hellmich M, Moinzabeh P, Rosenkranz S. Vardenafil for the treatment of raynaud phenomenon: a randomized, double-blind, placebo-controlled crossover study. Archives of International Medicine 2012;172(15):1182-4. [CLINICALTRIALS.GOV: NCT01291199] [EUROPEAN UNION CLINICAL TRIALS REGISTER: EUCTR2005-000295-41-DE] - PubMed
-
- Caglayan E, Axmann S, Huntgeburth M, Erdmann E, Rosenkranz S, Hellmich M et al. Vardenafil in patients with raynaud's syndrome. In: European Society of Cardiology and ESC Congress. Paris, France. Vol. 32(SUPPL. 1). 2011:655.
-
- Caglayan E, Axmann S, Huntgeburth M, Erdmann E, Rosenkranz S, Hellmich M, et al. Vardenafil improves clinical symptoms and digital blood flow in patients with primary and secondary raynaud syndrome - Results from a randomized controlled trial. In: American Heart Association's Scientific Sessions: Orlando and FL United States. Vol. 124(21 SUPPL. 1). 2011:https://www.ahajournals.org/browse/toc/circ/124/suppl_21/meeting-abstrac....
Fries 2005 {published data only}
-
- Fries R, Shariat K, Wilmowsky H, Bohm M. Sildenafil in the treatment of Raynaud's phenomenon resistant to vasodilatory therapy. Circulation 2005;112(19):2980-5. - PubMed
Herrick 2011 {published data only}
-
- Herrick AL, Van Den Hoogen F, Gabrielli A, Tamimi N, Reid C, O'Connell D, et al. Modified-release sildenafil reduces raynauds attack frequency in systemic sclerosis. In: American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting, ACR/ARHP 09. Atlanta and GA United States. Vol. 60(SUPPL. 10). 2009:472.
-
- Herrick AL, Van den Hoogen F, Gabrielli A, Tamimi N, Reid C, O'Connell D, et al. Modified-release sildenafil reduces Raynaud's phenomenon attack frequency in limited cutaneous systemic sclerosis. Arthritis and Rheumatism 2011;63(3):775-82. - PubMed
Laumann 2010 {unpublished data only}
-
- Laumann A. Tadalafil for the treatment of secondary Raynaud's phenomenon (unpublished data). www.clinicaltrials.gov/ct2/show/NCT00822354.
Pfizer 2018 {unpublished data only}
-
- Pfizer. A phase 2a randomized double-blinded, placebo and active controlled two cohort two doses cross-over multi-center clinical study to assess efficacy of a once daily administration of a phosphodiesterase 5 inhibitor (pf-00489791) for the treatment of vasospasm in primary and secondary Raynaud's phenomenon. www.clinicaltrials.gov/ct2/show/NCT01090492 2018. [EUROPEAN UNION CLINICAL TRIAL REGISTRY: EUCTR2010-019009-40-HU]
Schiopu 2009 {published data only}
-
- Schiopu E, Hsu VM, Impens AJ, Rothman JA, McCloskey DA, Wilson JE, et al. Randomized placebo-controlled trial of Tadalafil in Raynaud's phenomenon secondary to systemic sclerosis. Journal of Rheumatology 2009;36(10):2264-8. - PubMed
Shenoy 2010 {published data only}
-
- Shenoy P, Agarwal V, Misra R, Kumar S, Choudhary S, Jha L, et al. Tadalafil is effective in the management of resistant secondary Raynaud's phenomenon and improves endothelial function. In: British Society for Rheumatology and British Health Professionals in Rheumatology Annual Meetings 2009 and Rheumatology '09. Glasgow, United Kingdom. Vol. 48(SUPPL. 1). 2009:i.26 OP77.
-
- Shenoy PD, Kumar S, Jha LK, Choudhary SK, Singh U, Misra R, et al. Efficacy of tadalafil in secondary Raynaud's phenomenon resistant to vasodilator therapy: a double-blind randomized cross-over trial. Rheumatology 2010;49(12):2420-8. [CLINICALTRIALS.GOV: NCT00626665] - PubMed
References to studies excluded from this review
Bellando‐Randone 2010 {published data only}
-
- Bellando-Randone S, Miniati I, Guiducci S, Letizia CM, Blagojevic J, Fiori G, et al. Sildenafil increases vasodilation in systemic sclerosis: an interim analysis from a single centre pilot study [abstract #593]. In: Arthritis and Rheumatology. Vol. 62 Suppl 10. 2010:S248. [DOI: DOI: 10.1002/art.30166]
Friedman 2007 {published data only}
-
- Friedman EA, Harris PA, Wood AJ, Stein CM, Kurnik D. The effects of tadalafil on cold-induced vasoconstriction in patients with Raynaud's phenomenon. Clinical Pharmacology and Therapeutics 2007;81(4):503-9. - PubMed
Park 2013 {published data only}
-
- Park JK, Lee EY, Yoon MJ, Kim YK, Park CS, Giles JT, et al. Head to head comparison of Udenafil versus Amlodipine in the treatment of secondary Raynaud's phenomenon in the treatment of secondary Raynaud's Phenomenon: a double-blind, randomized cross-over study. In: Scientific Abstracts. 2013. - PubMed
Roustit 2018 {published data only}
-
- Cracowski JL, Roustit M, Mouhib M, Lotito A, Khouri C, Giai J et al. On demand sildenafl as a treatment of raynaud's phenomenon: A series of N-of-1 trials. In: 2nd Joint Meeting of the European Society for Microcirculation, ESM and European Vascular Biology Organisation and EVBO. Geneva Switzerland. Vol. 54(Supplement 1). 2017:11.
-
- Roustit M, Cracowski JL, Giai J, Subtil F, Gaget O, Mouhib M, et al. In: On-demand sildenafil as a treatment for raynaud's phenomenon: A series of N-of-1 trials. 5th Systemic Sclerosis World Congress. Bordeaux France. Vol. 3(Supplement 1). 2018:175-176.
-
- Roustit M, Gaget O, Mouhib M, Lotito A, Khouri C, Blaise S et al. On demand sildenafil as a treatment of raynaud's phenomenon: A series of n-of-1 trials. In: 13th Congress of the European Association for Clinical Pharmacology and Therapeutics and EACPT 2017. Prague Czechia. Vol. 39(8 Supplement 1). 2017:e4.
-
- Roustit M, Giai J, Gaget O, Khouri C, Mouhib M, Lotito A, et al. On-demand Sildenafil as a treatment for Raynaud phenomenon: a series of n-of-1 trials. Annals of Internal Medicine 2018;169(10):694-703. [CLINICALTRIALS.GOV: NCT02050360] [DOI: 10.7326/M18-0517] - DOI - PubMed
Watanabe 2004 {published data only}
-
- Watanabe H, Nishio S, Ohmae E, et al. Sildenafil improves fore arm blood flow in patients with Raynaud’s phenomenon (abstract P1207). In: European Heart Journal. Vol. 25. 2004:202.
References to studies awaiting assessment
NCT00528242 {unpublished data only}
-
- EUCTR2007-000397-23-DE (2007). A randomised, double blind, placebo-controlled, cross-over pilot study to examine the safety, tolerability and pharmacodynamic profile of repeat oral doses of SLx-2101 once daily for up to 14 days in patients with secondary Raynaud's disease. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2007-00039... (first received May 29, 2007).
-
- NCT00528242. Safety, tolerability, and pharmacodynamic profile of oral 2101 in secondary Raynaud's disease. clinicaltrials.gov/ct2/show/NCT00528242 (first received 12 September 2007).
NCT00707187 {unpublished data only}
-
- NCT00707187. Trial of IC351 in female scleroderma patients with Raynaud's and sexual dysfunction. clinicaltrials.gov/ct2/show/NCT00707187 (first received 30 June 2008).
Zamiri 2004 {published data only}
-
- Zamiri B, Koman AL, Smith BP, Smith TL, Holden M, Rushing J, et al. Double-blind, placebo-controlled trial of Sildenafil for the management of primary Raynaud's phenomenon (Abstract SAT0342). Annals of Rheumatic Diseases 2004;63 Suppl 1:484–5.
Additional references
Belch 2017
Dwan 2019
Egger 1997
-
- Egger M, Zellweger-Zahner T, Scheider M, Junker C, Lengeler AG. Language bias in randomised controlled trials published in English and German. Lancet 1997;350(907):326-9. - PubMed
García‐Carrasco 2008
-
- García-Carrasco M, Jiménez-Hernández M, Escárcega RO, Mendoza-Pinto C, Pardo-Santos R, Levy R. Treatment of Raynaud's phenomenon. Autoimmunity Reviews 2008;8(1):62-8. - PubMed
Goundry 2012
-
- Goundry B, Bell L, Langtree M, Moorthy A. Diagnosis and management of Raynaud’s phenomenon. BMJ 2012;344:e289. - PubMed
GRADE 2008
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 1 September 2021. Available at gradepro.org.
Hansteen 1976
-
- Hansteen V. Medical treatment in Raynaud's disease. Acta Chirurgica Scandinavica. Supplementum 1976;465:87-91. - PubMed
Herrick 2005
-
- Herrick AL. Pathogenesis of Raynaud's phenomenon. Rheumatology 2005;44(5):587-96. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cohrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
-
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook/.
Higgins 2022
-
- Higgins JPT, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Impens 2011
Kowal‐Bielecka 2017
-
- Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Annals of Rheumatic Diseases 2017;76(8):1327-39. [PMID: PMID: 27941129] - PubMed
Lefebvre 2017
-
- Lefebvre C, Manheimer M, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
LeRoy 1988
-
- LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. Journal of Rheumatology 1988;15(2):202-5. [PMID: ] - PubMed
LeRoy 1992
-
- LeRoy EC, Medsger TA. Raynaud's phenomenon: a prosposal for classification. Clinical and Experimental Rheumatology 1992;10:485-8. - PubMed
Levien 2010
Masi 1980
-
- Masi AT, Rodnan GP, Medsger TA Jr. ACR Preliminary criteria for the classification of systemic sclerosis (scleroderma): Special article. Arthritis and Rheumatism 1980;23(5):581-90. - PubMed
Maundrell 2015
-
- Maundrell A, Proudman SM. Epidemiology of Raynaud’s phenomenon. In: Raynaud's Phenomenon. 1st edition. New York (NY): Springer, 2015:21-35.
Merkel 2002
-
- Merkel PA, Herlyn K, Martin RW, Anderson JJ, Mayes MD, Bell P, et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud's phenomenon. Arthritis and Rheumatism 2002;46(9):2410-20. - PubMed
Ortonne 1989
-
- Ortonne JP, Torzuoli C, Dujardin P, Fraitag B. Ketanserin in the treatment of systemic sclerosis: a double‐blind controlled trial. British Journal of Dermatology 1989;120(2):261-6. - PubMed
RevMan Web 2022 [Computer program]
-
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rirash 2017
Roustit 2013
-
- Roustit M, Blaise S, Allanore Y, Carpentier PH, Caglayan E, Cracowski JL. Phosphodiesterase-5 inhibitors for the treatment of secondary Raynaud's phenomenon: systematic review and meta-analysis of randomised trials. Annals of Rheumatic Disease 2013;72(10):1696-9. [DOI: 10.1136/annrheumdis-2012-202836] - DOI - PubMed
Schulz 2010
Schünemann 2017
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Available from: www.training.cochrane.org/handbook.
Wigley 2002
-
- Wigley FM. Raynaud's Phenomenon. New England Journal of Medicine 2002;347(13):1001-8. - PubMed
References to other published versions of this review
Rirash 2021
-
- Rirash F, Tingey PC, Harding SE, Maxwell LJ, Ghogomu ET, Maltez N, et al. Drug interventions versus placebo for the treatment of Raynaud’s phenomenon: generic protocol. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD011813. [DOI: 10.1002/14651858.CD011813.pub2] - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

