Changes Induced by Early Hand-Arm Bimanual Intensive Therapy Including Lower Extremities in Young Children With Unilateral Cerebral Palsy: A Randomized Clinical Trial
- PMID: 37930692
- PMCID: PMC10628844
- DOI: 10.1001/jamapediatrics.2023.4809
Changes Induced by Early Hand-Arm Bimanual Intensive Therapy Including Lower Extremities in Young Children With Unilateral Cerebral Palsy: A Randomized Clinical Trial
Erratum in
-
Errors in Abstract and Author Name.JAMA Pediatr. 2024 Feb 1;178(2):206. doi: 10.1001/jamapediatrics.2023.6170. JAMA Pediatr. 2024. PMID: 38109107 Free PMC article. No abstract available.
Abstract
Importance: Intensive interventions are provided to young children with unilateral cerebral palsy (UCP), classically focused on the upper extremity despite the frequent impairment of gross motor function. Hand-Arm Bimanual Intensive Therapy Including Lower Extremities (HABIT-ILE) effectively improves manual dexterity and gross motor function in school-aged children.
Objective: To verify if HABIT-ILE would improve manual abilities in young children with UCP more than usual motor activity.
Design, setting, and participants: This prospective randomized clinical trial (November 2018 to December 2021), including 2 parallel groups and a 1:1 allocation, recruitment took place at European university hospitals, cerebral palsy specialized centers, and spontaneous applications at 3 sites: Brussels, Belgium; Brest, France; and Pisa, Italy. Matched (age at inclusion, lesion type, cause of cerebral palsy, and affected side) pairs randomization was performed. Young children were assessed at baseline (T0), 2 weeks after baseline (T1), and 3 months after baseline (T2). Health care professionals and assessors of main outcomes were blinded to group allocation. At least 23 young children (in each group) aged 12 to 59 months with spastic/dyskinetic UCP and able to follow instructions were needed. Exclusion criteria included uncontrolled seizures, scheduled botulinum toxin injections, orthopedic surgery scheduled during the 6 months before or during the study period, severe visual/cognitive impairments, or contraindications to magnetic resonance imaging.
Interventions: Two weeks of usual motor activity including usual rehabilitation (control group) vs 2 weeks (50 hours) of HABIT-ILE (HABIT-ILE group).
Main outcomes and measures: Primary outcome: Assisting Hand Assessment (AHA); secondary outcomes: Gross Motor Function Measure-66 (GMFM-66), Pediatric Evaluation of Disability Inventory-Computer Adaptive Test (PEDI-CAT), and Canadian Occupational Performance Measure (COPM).
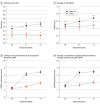
Results: Of 50 recruited young children (26 girls [52%], median age; 35.3 months for HABIT-ILE group; median age, 32.8 months for control group), 49 were included in the final analyses. Change in AHA score from T0 to T2 was significantly greater in the HABIT-ILE group (adjusted mean score difference [MD], 5.19; 95% CI, 2.84-7.55; P < .001). Changes in GMFM-66 (MD, 4.72; 95% CI, 2.66-6.78), PEDI-CAT daily activities (MD, 1.40; 95% CI, 0.29-2.51), COPM performance (MD, 3.62; 95% CI, 2.91-4.32), and satisfaction (MD, 3.53; 95% CI, 2.70-4.36) scores were greater in the HABIT ILE group.
Conclusions and relevance: In this clinical trial, early HABIT-ILE was shown to be an effective treatment to improve motor performance in young children with UCP. Moreover, the improvements had an impact on daily life activities of these children.
Trial registration: ClinicalTrials.gov Identifier: NCT04020354.
Conflict of interest statement
Figures
Comment in
-
Critically appraised paper: Hand-arm bimanual intensive therapy including lower extremities (HABIT-ILE) improves bi-manual performance and gross motor function in pre-school children with unilateral cerebral palsy [synopsis].J Physiother. 2024 Apr;70(2):150. doi: 10.1016/j.jphys.2024.02.005. Epub 2024 Mar 11. J Physiother. 2024. PMID: 38472054 No abstract available.
References
-
- Straub K, Obrzut JE. Effects of cerebral palsy on neuropsychological function. J Dev Phys Disabil. 2009;21(2):153-167. doi:10.1007/s10882-009-9130-3 - DOI

