Thermal stability and storage of human insulin
- PMID: 37930742
- PMCID: PMC10627263
- DOI: 10.1002/14651858.CD015385.pub2
Thermal stability and storage of human insulin
Abstract
Background: Health authorities stress the temperature sensitivity of human insulin, advising protection from heat and freezing, with manufacturers suggesting low-temperature storage for intact vials, and once opened, storage at room temperature for four to six weeks, though usage time and maximum temperature recommendations vary. For human insulin, the recommendations of current shelf life in use may range from 10 to 45 days, and the maximum temperature in use varies between 25 °C and 37 °C. Optimal cold-chain management of human insulin from manufacturing until the point of delivery to people with diabetes should always be maintained, and people with diabetes and access to reliable refrigeration should follow manufacturers' recommendations. However, a growing segment of the diabetes-affected global population resides in challenging environments, confronting prolonged exposure to extreme heat due to the climate crisis, all while grappling with limited access to refrigeration.
Objectives: To analyse the effects of storing human insulin above or below the manufacturers' recommended insulin temperature storage range or advised usage time, or both, after dispensing human insulin to people with diabetes.
Search methods: We used standard, extensive Cochrane search methods. The latest search date was 12 July 2023.
Selection criteria: We included clinical and laboratory studies investigating the storage of human insulin above or below manufacturers' recommended temperature storage range, advised usage time, or both.
Data collection and analysis: We used standard Cochrane methods. We used GRADE to assess the certainty of evidence for the clinical study. Most information emerged from in vitro studies, mainly from pharmaceutical companies. There is no validated risk of bias and certainty of evidence rating for in vitro studies. We thus presented a narrative summary of the results.
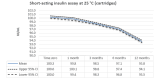
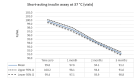
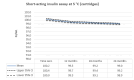
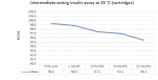
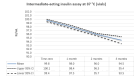
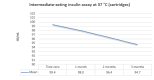
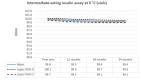
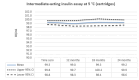
Main results: We included 17 eligible studies (22 articles) and additional information from pharmaceutical companies. Pilot clinical study One pilot clinical study investigated temperature conditions for insulin stored for six weeks in an unglazed clay pot with temperatures ranging between 25 °C and 27 °C. The mean fall in plasma glucose in eight healthy volunteers after clay pot-stored insulin injection was comparable to refrigerator-stored insulin injection (very low-certainty evidence). In-vitro studies Nine, three and four laboratory studies investigated storage conditions for insulin vials, insulin cartridges/pens and prefilled plastic syringes, respectively. The included studies reported numerous methods, laboratory measurements and storage conditions. Three studies on prefilled syringes investigating insulin potency at 4 °C up to 23 °C for up to 28 days showed no clinically relevant loss of insulin activity. Nine studies examined unopened vials and cartridges. In studies with no clinically relevant loss of insulin activity for human short-acting insulin (SAI), intermediate-acting insulin (IAI) and mixed insulin (MI) temperatures ranged between 28.9 °C and 37 °C for up to four months. Two studies reported up to 18% loss of insulin activity after one week to 28 days at 37 °C. Four studies examined opened vials and cartridges at up to 37 °C for up to 12 weeks, indicating no clinically relevant reduction in insulin activity. Two studies analysed storage conditions for oscillating temperatures ranging between 25 °C and 37 °C for up to 12 weeks and observed no loss of insulin activity for SAI, IAI and MI. Four studies, two on vials (including one on opened vials), and two on prefilled syringes, investigated sterility and reported no microbial contamination. Data from pharmaceutical companies Four manufacturers (BIOTON, Eli Lilly and Company, Novo Nordisk and Sanofi) provided previously unreleased human insulin thermostability data mostly referring to unopened containers (vials, cartridges). We could not include the data from Sanofi because the company announced the permanent discontinuation of the production of human insulins Insuman Rapid, Basal and Comb 25. BIOTON provided data on SAI after one, three and six months at 25 °C: all investigated parameters were within reference values, and, compared to baseline, loss of insulin activity was 1.1%, 1.0% and 1.7%, respectively. Eli Lilly and Company provided summary data: at below 25 °C or 30 °C SAI/IAI/MI could be stored for up to 25 days or 12 days, respectively. Thereafter, patient in-use was possible for up to 28 days. Novo Nordisk provided extensive data: compared to baseline, after three and six months at 25 °C, loss of SAI activity was 1.8% and 3.2% to 3.5%, respectively. Loss of IAI activity was 1.2% to 1.9% after three months and 2.0% to 2.3% after six months. Compared to baseline, after one, two and three months at 37 °C, loss of SAI activity was 2.2% to 2.8%, 5.7% and 8.3% to 8.6%, respectively. Loss IAI activity was 1.4% to 1.8%, 3.0% to 3.8% and 4.7% to 5.3%, respectively. There was no relevant increase in insulin degradation products observed. Up to six months at 25 °C and up to two months at 37 °C high molecular weight proteins were within specifications. Appearance, visible particles or macroscopy, particulate matter, zinc, pH, metacresol and phenol complied with specifications. There were no data for cold environmental conditions and insulin pumps.
Authors' conclusions: Under difficult living conditions, pharmaceutical companies' data indicate that it is possible to store unopened SAI and IAI vials and cartridges at up to 25 °C for a maximum of six months and at up to 37 °C for a maximum of two months without a clinically relevant loss of insulin potency. Also, oscillating temperatures between 25 °C and 37 °C for up to three months result in no loss of insulin activity for SAI, IAI and MI. In addition, ambient temperature can be lowered by use of simple cooling devices such as clay pots for insulin storage. Clinical studies on opened and unopened insulin containers should be performed to measure insulin potency and stability after varying storage conditions. Furthermore, more data are needed on MI, insulin pumps, sterility and cold climate conditions.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
BR: none.
BB: none.
MIM: none.
The authors are editors for the Cochrane Metabolic and Endocrine Disorders group, but they had no role in the editorial processing of this review.
Figures







Update of
References
References to studies included in this review
Al Shaibi 1999 {published data only}
-
- Al Shaibi K, Falata W, Sayes N, Al Shareef M, Al Taweel M, Abozenadah A, et al. Storing insulin in a clay pot in the desert causes no loss of activity: a preliminary report. Annals of Saudi Medicine 1999;19(6):547-9. - PubMed
Baechler 2020 {published data only}
Delbeck 2021a {published data only}
Dunbar 1986 {published data only}
Gregory 1991 {published data only}
Kaufmann 2021 {published data only}
Koffler 1992 {published data only}
Kongmalai 2021 {published data only}
Lull 2013 {published data only}
Moses 2019 {published data only}
-
- Moses A, Bjerrum J, Hach M, Waehrens LH, Toft AD. Concentrations of intact insulin concurs with FDA and EMA standards when measured by HPLC in different parts of the distribution cold chain. Journal of Diabetes Science and Technology 2019;13(1):55-9. [DOI: 10.1177/1932296818783783] - DOI - PMC - PubMed
Pendsey 2023 {published data only}
Schrader 1985 {published data only}
-
- Schrader E, Pfeiffer EF. The influence of motion and temperature upon the aggregational behavior of soluble insulin formulations investigated by high-performance liquid-chromatography. Journal of Liquid Chromatography 1985;8(6):1139-57. [DOI: 10.1080/01483918508067133] - DOI
Shnek 1998 {published data only}
Silva 2013 {published data only}
-
- Silva MA, Chuong M, Kerr S, Cabrera A. Stability of two long-acting insulin formulations after 28 days. Journal of Pharmacy Practice & Research 2013;43(1):37-40. [DOI: 10.1002/j.2055-2335.2013.tb00213.x] - DOI
Silva‐Jr 2022 {published data only}
Tarr 1991 {published data only}
-
- Tarr BD, Campbell RK, Workman TM. Stability and sterility of biosynthetic human insulin stored in plastic insulin syringes for 28 days. American Journal of Hospital Pharmacy 1991;48(12):2631-4. - PubMed
Vimalavathini 2009 {published data only}
-
- Vimalavathini R, Gitanjali B. Effect of temperature on the potency & pharmacological action of insulin. Indian Journal of Medical Research 2009;130(2):166-9. - PubMed
References to studies excluded from this review
Albergo 1992 {published data only}
Allen 1982 {published data only}
-
- Allen SC. A cool storage pot for insulin in rural Africa. Medical Journal of Zambia 1982;16(4):83-4. - PubMed
Bishop 1970 {published data only}
Braune 2019 {published data only}
Casey 1983 {published data only}
-
- Casey CA. Storage of insulin stirs controversy. Rn National Magazine for Nurses 1983;46(7):73.
Davis 2010 {published data only}
-
- Davis SR, Anderson EA. Creation of a temperature stability database for refrigerated medications. Journal of Pharmacy Practice and Research 2010;40(1):31-5. [DOI: 10.1002/j.2055-2335.2010.tb00722.x] - DOI
Delbeck 2021b {published data only}
Dokken 2017 {published data only}
Fisch 1987 {published data only}
-
- Fisch A, Leblanc H, Cisse H, Pichard E, Zirinis P. Stability of ordinary insulins under African climatic conditions. Medecine d'Afrique Noire 1987;34(12):1043-8.
Gill 2000 {published data only}
Gill 2002 {published data only}
Gilligan 2021 {published data only}
Grajower 2003 {published data only}
Grajower 2014 {published data only}
-
- Flood TM. In response: how long should insulin be used once a vial is opened? Endocrine Practice 2015;21(2):213. - PubMed
-
- Grajower MM. How long can a vial of insulin be used after it is started: where are we 10 years later? Endocrine Practice 2014;20(2):188-90. [DOI: 10.4158/EP13427.CO] - DOI - PubMed
Grant 1946 {published data only}
-
- Grant RL. Loss of potency of commercial insulin stored at room temperature. Federation Proceedings 1946;5(1 Pt 2):181. - PubMed
Grau 1985 {published data only}
-
- Grau U. Chemical stability of insulin in a delivery system environment. Diabetologia 1985;28(7):458-63. - PubMed
Havlova 1966 {published data only}
-
- Havlova M, Nobilis M. Undesirable factors influencing the stability of insulin preparations [NEŽÁDOUCÍ VLIVY PŮSOBÍCÍ NA STABILITU INSULINOVÝCH PREPARÁTŮ]. Ceskoslovenska Farmacie 1966;15(9):504-5. - PubMed
Hearnshaw 2009 {published data only}
Herr 2014 {published data only}
Hildebrandt 1986 {published data only}
-
- Hildebrandt R, Jannasch R, Lotz U. On the stability of insulin solutions in syringes. Zeitschrift für Klinische Medizin 1986;41(1):51-3.
Hildebrandt 1987 {published data only}
-
- Hildebrandt R, Rafalski I. Stability and sterility of neutral insulin after opening. Zeitschrift für Klinische Medizin 1987;42(14):1279-81.
Kalra 2012 {published data only}
-
- Kalra S, Kalra B. Storage of insulin in rural areas. Journal of Academy of Medical Sciences 2012;2(2):88-9. [DOI: 10.4103/2249-4855.118669] - DOI
Kasmer 1986 {published data only}
Keller 2003 {published data only}
-
- Keller KJ. Insulin storage and stability update. South Dakota Journal of Medicine 2003;56(8):303-4. - PubMed
Khurana 2019 {published data only}
-
- Khurana G, Gupta VK. Effect on insulin upon storage in extreme climatic conditions (temperature and pressure) and their preventive measures. Journal of Social Health and Diabetes 2019;7(1):6-10. [DOI: 10.1055/s-0039-1692371] - DOI
Kircher 1990 {published data only}
-
- Kircher W. Drug information. Instructions for storage and administration. Insulin preparations [Arzneimittelanwendung. Aufbewahrungs- und Abgabehinweise. Insulinpräparate]. Medizinische Monatsschrift für Pharmazeuten 1990;13(10):318-20. - PubMed
Kongmalai 2022 {published data only}
-
- Kongmalai T, Orarachin P, Dechates B, Chanphibun P, Junnu S, Srisawat C, et al. The effect of high temperature on the stability of basal insulin in a pen: a randomized controlled, crossover, equivalence trial. BMJ Open Diabetes Research & Care 2022;10(6):1-8. [DOI: 10.1136/bmjdrc-2022003105] - DOI - PMC - PubMed
Kristensen 2011 {published data only}
Krogh 1928 {published data only}
Levine 1970 {published data only}
-
- Levine R, Mahler R, Moss JM. The management of diabetes. 7. Characteristics of the available insulins. Problems in insulin storage. American Family Physician/GP 1970;2(5):151-63. - PubMed
Lopez 2014 {published data only}
Minuto 2010 {published data only}
Mitchell 2012 {published data only}
Mokta 2014 {published data only}
-
- Mokta JK, Kalra S. Insulin storage in the Upper Himalayas. Rural and Remote Health 2014;14(2983):1-2. - PubMed
Nassar 2010 {published data only}
Ogle 2016 {published data only}
Oliva 2009 {published data only}
Pendsey 2006 {published data only}
-
- Pendsey S. Keeping insulin cool naturally – the DREAM Trust storage system. Diabetes Voice 2006;51(Special Issue):19.
Perriello 1988 {published data only}
-
- Perriello G, Torlone E, Di Santo S, Fanelli C, De Feo P, Santeusanio F, et al. Effect of storage temperature of insulin on pharmacokinetics and pharmacodynamics of insulin mixtures injected subcutaneously in subjects with type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1988;31(11):811-5. [DOI: 10.1007/BF00277482] - DOI - PubMed
Peters 1987 {published data only}
-
- Peters AL, Davidson MB. Effect of storage on action of NPH and regular insulin mixtures. Diabetes Care 1987;10(6):799-800. - PubMed
Pfützner 2016 {published data only}
-
- Pfützner A, Nagar R. Technology solutions to Increase insulin stability in case of extreme weather conditions. Diabetes Technology & Therapeutics 2016;18:A122-3.
Pfützner 2017 {published data only}
Pfützner 2018a {published data only}
-
- Pfützner A, Reeh W, Nagar R, Rose D. The Vivi-Cap stabilizes insulin in extreme temperature conditions. Diabetes Technology & Therapeutics 2018;20:A140-1.
Pfützner 2018b {published data only}
-
- Pfützner A, Reeh W, Strobl S, Rose D. ViViCap – reliable, reusable protection against insulin degradation in high and low ambient temperatures. Diabetes Stoffwechsel und Herz 2018;27(6):307-11.
Pfützner 2018c {published data only}
-
- Pfützner A, Nagar R, Reeh W. A reusable cap for disposable insulin pens protects insulin against temperature-induced degradation. Diabetes 2018;67(Suppl 1):2. [DOI: 10.2337/db18-2281-PUB] - DOI
Pingel 1972 {published data only}
Plager 2017 {published data only}
Pryce 2009 {published data only}
Puepet 2007 {published data only}
-
- Puepet FH, Mijinyawa BB, Akogu IA. Insulin storage by patients with diabetes in Jos, Nigeria. Journal of Medical Tropics 2007;9:37-40.
Rathod 1985 {published data only}
Richards 2013 {published data only}
Santiago 2002 {published data only}
Seyoum 1997 {published data only}
-
- Seyoum B, Abdulkadir J, Feleke Y, Worku Y. Insulin storage by diabetic patients in Ethiopia. Diabetologia 1997;40:2568.
Steil 1988 {published data only}
Stephenson 1960 {published data only}
Stephenson 2018 {published data only}
-
- Stephenson J. Domestic refrigerators 'may pose risk to insulin quality'. Nursing Times 2018;114(10):46.
Storvick 1968 {published data only}
TCTR20210611002 {published data only}
-
- TCTR20210611002. Efficacy of in-used insulin pen kept at 37 Celsius for 21 days on mean daily glucose measured by continuous glucose monitoring compare with the effect of newly used insulin pen, the equivalence trial. www.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20210611002 (accessed 3 January 2022).
Thirunavukkarasu 1968 {published data only}
-
- Thirunavukkarasu K. Soluble insulin in the tropics. Ceylon Medical Journal 1968;13(3):150-3. - PubMed
Vereshchetin 2016 {published data only}
Virmani 2020 {published data only}
-
- Virmani A, Avni TC. A case for expanding thermochromic vial monitor technology to insulin and other biologics. Indian Pediatrics 2020;57(1):17-9. - PubMed
Westphal 2010 {published data only}
Yun‐Chu 2017 {published data only}
-
- Yun-Chu C, Francoise K, Karen EJ, Asel S. Thermal stability, storage, release and delivery of insulin – a protection through sol-gel tailored silica. European Biophysics Journal 2017;46(Suppl 1):S320.
Zell 1983 {published data only}
-
- Zell M, Paone RP. Stability of insulin in plastic syringes. American Journal of Hospital Pharmacy 1983;40(4):637-8. - PubMed
Zhao 2015 {published data only}
-
- Zhao Xiaolei, Zheng Silin. Research progress on period of validity and storage conditions of opened insulin [胰岛素开启后有效期和贮藏条件的研究进展]. Chinese Nursing Research 2015;29(11A):3848-50. [DOI: 10.3969/j.issn.1009-6493.2015.31.003] - DOI
Additional references
ADA 2003
-
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(Suppl 1):S5-20. - PubMed
ADA 2017
-
- American Diabetes Association. Standards of medical care in diabetes – 2017. Diabetes Care 2017;40(Suppl 1):S11-33.
ADA 2021
-
- American Diabetes Association. Insulin storage and syringe safety. www.diabetes.org/healthy-living/medication-treatments/insulin-other-inje... (accessed 15 October 2021).
Ahmad 2003
Arora 2004
Atwoli 2021
Bahendeka 2019
Basu 2019
Beran 2006
Beran 2016
Brange 1987
-
- Brange J. Galenics of Insulin. Heidelberg: Springer-Verlag, 1987.
Brange 1997
Burki 2022
-
- Burki T. Hot topics on insulin access: the issue of thermostability. Lancet. Diabetes & Endocrinology 2022;10(6):389-90. [DOI: 10.1016/ S2213-8587(22)00140-1] - PubMed
Calo 2016
Chance 1993
Danne 2018
Delbeck 2020a
Delbeck 2020b
-
- Delbeck S, Heise HM. FT-IR versus EC-QCL spectroscopy for biopharmaceutical quality assessment with focus on insulin-total protein assay and secondary structure analysis using attenuated total reflection. Analytical and Bioanalytical Chemistry 2020;412(19):4647-58. [DOI: 10.1007/s00216-020-02718-1] - DOI - PMC - PubMed
Derewenda 1989
Devi 2021
-
- Devi S. Cold comfort: getting insulin to those who need it. Lancet 2021;398(10313):1791. - PubMed
Ebi 2021
European Pharmacopoeia 2023
-
- European Pharmacopoeia (Ph. Eur), 11th Edition. Strasbourg: European Pharmacopoeia Commission, European Directorate for the Quality of Medicines (EDQM); 2023.
Farid 1989
Fisher 1986
Flemyng 2023
Flood 2015
-
- Flood TM. In response: how long should insulin be used once a vial is opened? Endocrine Practice 2015;21(2):213. - PubMed
Fullerton 2018
-
- Fullerton B, Siebenhofer A, Jeitler K, Horvath K, Semlitsch T, Berghold A, et al. Short-acting insulin analogues versus regular human insulin for adult, non-pregnant persons with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2018, Issue 12. Art. No: CD013228. [DOI: 10.1002/14651858.CD013228] - DOI - PMC - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 29 June 2023. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Grajower 2003
Grajower 2014
-
- Grajower M. How long can a vial of insulin be used after it is started: where are we 10 years later? Endocrine Practice 2014;20(2):188-90. [DOI: 10.4158/EP13427.CO] - DOI - PubMed
Groenning 2009
Heinemann 2021
Hemmingsen 2021
Herr 2014
Higgins 2022a
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Higgins 2022b
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Huus 2005
Huus 2006
IDF 2021
-
- International Diabetes Federation. IDF Diabetes Atlas 2021 (IDF Atlas 10th edition). diabetesatlas.org/en/resources (accessed 18 December 2021).
Kaufmann 2021
Khurana 2019
-
- Khurana G, Gupta VK. Effect on insulin upon storage in extreme climatic conditions (temperature and pressure) and their preventive measures. Journal of Social Health and Diabetes 2019;7(1):6-10. [DOI: 10.1055/s-0039-1692371] - DOI
Kurouski 2012
Lilly 2023
-
- Humulin R – insulin human injection, solution. uspl.lilly.com/humulinru100/humulinru100.html#ug0 (assessed 25 June 2023).
Mori 2021
Novo Nordisk 2020a
-
- Insulatard: EPAR – product information. www.ema.europa.eu/documents/product-information/insulatard-epar-product-... (assessed 29 June 2023).
Novo Nordisk 2020b
-
- Actrapid: EPAR – product information. www.ema.europa.eu/documents/product-information/actrapid-epar-product-in... (assessed 29 June 2023).
Ogle 2016
Ohno 2019
Page 2021
Pande 2022
-
- Pande AR, Thakur AK. Perceptions of alternative methods of insulin storage: an ethical dilemma. Lancet. Diabetes & Endocrinology 2022;10(8):558. - PubMed
Pingel 1972
Pryce 2009
Quijal‐Zamorano 2021
-
- Quijal-Zamorano M, Martinez-Solanas E, Achebak H, Petrova D, Robine JM, Herrmann FR, et al. Seasonality reversal of temperature attributable mortality projections due to previously unobserved extreme heat in Europe. Lancet Planet Health 2021;5(9):e573. [DOI: 10.1016/S2542-5196(21)00211-4] - DOI - PubMed
Sanofi 2023a
-
- Editor. Sanofi to discontinue Insuman® insulins over upcoming months. diabetestimes.co.uk/sanofi-to-discontinue-insuman-insulins-over-upcoming... (assessed 11 June 2023).
Sanofi 2023b
-
- Information letter from Sanofi-Aventis Deutschland GmbH on the worldwide discontinuation of the production of the medicinal products Insuman basal, Insuman rapid and Insuman comb25 [Informationsschreiben der Sanofi-Aventis Deutschland GmbH zur weltweiten Einstellung der Produktion der Arzneimittel INSUMAN BASAL, INSUMAN RAPID und INSUMAN COMB25]. www.bfarm.de/SharedDocs/Arzneimittelzulassung/Lieferengpaesse/DE/2023/in... (assessed 29 June 2023).
Schünemann 2022
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Semlitsch 2020
-
- Semlitsch T, Engler J, Siebenhofer A, Jeitler K, Berghold A, Horvath K. (Ultra-)long-acting insulin analogues versus NPH insulin (human isophane insulin) for adults with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD005613. [DOI: 10.1002/14651858.CD005613.pub4] - DOI - PMC - PubMed
Shnek 1998
Smith 1985
-
- Smith HW Jr, Atkins LM, Binkley DA, Richardson WG, Miner DJ. A universal HPLC determination of insulin potency. Journal of Liquid Chromatography 1985;8(3):419-39.
Sterne 2016
Storvick 1968
Sun 2022
-
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice 2022;183:109119. [DOI: 10.1016/j.diabres.2021.109119] - DOI - PMC - PubMed
Taerahkun 2022
USP 2016
-
- US Pharmacopeia. Insulin Human Drug Products 100 IU/ml. Rockville, MD: United States Pharmacopeial Convention; 2016.
Vimalavathini 2009
-
- Vimalavathini R, Gitanjali B. Effect of temperature on the potency & pharmacological action of insulin. Indian Journal of Medical Research 2009;130(2):166-9. - PubMed
Weiss 2013
-
- Weiss M. Design of ultra-stable insulin analogues for the developing world. Journal of Health Specialities 2013;1(2):59-70.
Westphal 2010
WHO 1999
-
- World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. In: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva (Switzerland): World Health Organization, 1999:1-59.
WHO 2011
-
- WHO Technical Report Series, No 961. Model guidance for the storage and transport of time- and temperature-sensitive pharmaceutical products. www.who.int/medicines/areas/quality_safety/quality_assurance/ModelGuidan... (accessed 15 October 2021).
WHO 2020
-
- World Health Organization. Diagnosis and management of type 2 diabetes. apps.who.int/iris/rest/bitstreams/1274478/retrieve (accessed 11 August 2023).
WHO EML 2021
-
- World Health Organization. World Health Organization Model List of Essential Medicines – 22nd List (2021). apps.who.int/iris/rest/bitstreams/1374779/retrieve (accessed 15 October 2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

