Guanine-containing ssDNA and RNA induce dimeric and tetrameric structural forms of SAMHD1
- PMID: 37930833
- PMCID: PMC10711556
- DOI: 10.1093/nar/gkad971
Guanine-containing ssDNA and RNA induce dimeric and tetrameric structural forms of SAMHD1
Abstract
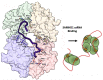
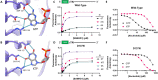
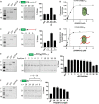
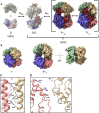
The dNTPase activity of tetrameric SAM and HD domain containing deoxynucleoside triphosphate triphosphohydrolase 1 (SAMHD1) plays a critical role in cellular dNTP regulation. SAMHD1 also associates with stalled DNA replication forks, DNA repair foci, ssRNA and telomeres. The above functions require nucleic acid binding by SAMHD1, which may be modulated by its oligomeric state. Here we establish in cryo-EM and biochemical studies that the guanine-specific A1 activator site of each SAMHD1 monomer is used to target the enzyme to guanine nucleotides within single-stranded (ss) DNA and RNA. Remarkably, nucleic acid strands containing a single guanine base induce dimeric SAMHD1, while two or more guanines with ∼20 nucleotide spacing induce a tetrameric form. A cryo-EM structure of ssRNA-bound tetrameric SAMHD1 shows how ssRNA strands bridge two SAMHD1 dimers and stabilize the structure. This ssRNA-bound tetramer is inactive with respect to dNTPase and RNase activity.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




Update of
-
Guanine-containing ssDNA and RNA induce dimeric and tetrameric SAMHD1 in cryo-EM and binding studies.bioRxiv [Preprint]. 2023 Jun 15:2023.06.15.544806. doi: 10.1101/2023.06.15.544806. bioRxiv. 2023. Update in: Nucleic Acids Res. 2023 Dec 11;51(22):12443-12458. doi: 10.1093/nar/gkad971. PMID: 37398126 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

