Spatial and Single-Cell Transcriptomics Reveal a Cancer-Associated Fibroblast Subset in HNSCC That Restricts Infiltration and Antitumor Activity of CD8+ T Cells
- PMID: 37930937
- PMCID: PMC10790129
- DOI: 10.1158/0008-5472.CAN-23-1448
Spatial and Single-Cell Transcriptomics Reveal a Cancer-Associated Fibroblast Subset in HNSCC That Restricts Infiltration and Antitumor Activity of CD8+ T Cells
Abstract
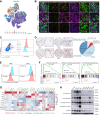
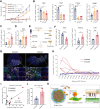
Although immunotherapy can prolong survival in some patients with head and neck squamous cell carcinoma (HNSCC), the response rate remains low. Clarification of the critical mechanisms regulating CD8+ T-cell infiltration and dysfunction in the tumor microenvironment could help maximize the benefit of immunotherapy for treating HNSCC. Here, we performed spatial transcriptomic analysis of HNSCC specimens with differing immune infiltration and single-cell RNA sequencing of five pairs of tumor and adjacent tissues, revealing specific cancer-associated fibroblast (CAF) subsets related to CD8+ T-cell infiltration restriction and dysfunction. These CAFs exhibited high expression of CXCLs (CXCL9, CXCL10, and CXCL12) and MHC-I and enrichment of galectin-9 (Gal9). The proportion of MHC-IhiGal9+ CAFs was inversely correlated with abundance of a TCF1+GZMK+ subset of CD8+ T cells. Gal9 on CAFs induced CD8+ T-cell dysfunction and decreased the proportion of tumor-infiltrating TCF1+CD8+ T cells. Collectively, the identification of MHC-IhiGal9+ CAFs advances the understanding of the precise role of CAFs in cancer immune evasion and paves the way for more effective immunotherapy for HNSCC.
Significance: Spatial analysis identifies IFN-induced MHC-IhiGal9+ CAFs that form a trap for CD8+ T cells, providing insights into the complex networks in the tumor microenvironment that regulate T-cell infiltration and function.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures





References
-
- Carlisle JW, Steuer CE, Owonikoko TK, Saba NF. An update on the immune landscape in lung and head and neck cancers. CA Cancer J Clin 2020;70:505–17. - PubMed
-
- Park J, Hsueh PC, Li Z, Ho PC. Microenvironment-driven metabolic adaptations guiding CD8+ T-cell antitumor immunity. Immunity 2023;56:32–42. - PubMed
-
- Davidson S, Coles M, Thomas T, Kollias G, Ludewig B, Turley S, et al. . Fibroblasts as immune regulators in infection, inflammation, and cancer. Nat Rev Immunol 2021;21:704–17. - PubMed
Publication types
MeSH terms
Grants and funding
- 82173148/National Natural Science Foundation of China (NSFC)
- 82273267/National Natural Science Foundation of China (NSFC)
- 82272983/National Natural Science Foundation of China (NSFC)
- SHSMU-ZLCX20212300/Innovative Research Team of High-level Local University in Shanghai (Innovative Research Team of High-level Local Universities in Shanghai)
- 19MC1910600/Shanghai Clinical Research Center for Oral Diseases
LinkOut - more resources
Full Text Sources
Medical
Research Materials

