Caspase cleavage of gasdermin E causes neuronal pyroptosis in HIV-associated neurocognitive disorder
- PMID: 37931057
- PMCID: PMC10834258
- DOI: 10.1093/brain/awad375
Caspase cleavage of gasdermin E causes neuronal pyroptosis in HIV-associated neurocognitive disorder
Abstract
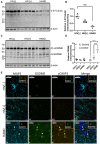
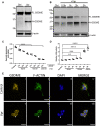
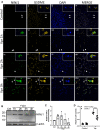
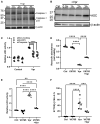
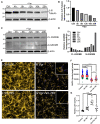
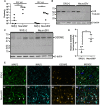
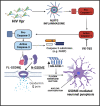
Despite effective antiretroviral therapies, 20-30% of persons with treated HIV infection develop a neurodegenerative syndrome termed HIV-associated neurocognitive disorder (HAND). HAND is driven by HIV expression coupled with inflammation in the brain but the mechanisms underlying neuronal damage and death are uncertain. The inflammasome-pyroptosis axis coordinates an inflammatory type of regulated lytic cell death that is underpinned by the caspase-activated pore-forming gasdermin proteins. The mechanisms driving neuronal pyroptosis were investigated herein in models of HAND, using multi-platform molecular and morphological approaches that included brain tissues from persons with HAND and simian immunodeficiency virus (SIV)-infected non-human primates as well as cultured human neurons. Neurons in the frontal cortices from persons with HAND showed increased cleaved gasdermin E (GSDME), which was associated with β-III tubulin degradation and increased HIV levels. Exposure of cultured human neurons to the HIV-encoded viral protein R (Vpr) elicited time-dependent cleavage of GSDME and Ninjurin-1 (NINJ1) induction with associated cell lysis that was inhibited by siRNA suppression of both proteins. Upstream of GSDME cleavage, Vpr exposure resulted in activation of caspases-1 and 3. Pretreatment of Vpr-exposed neurons with the caspase-1 inhibitor, VX-765, reduced cleavage of both caspase-3 and GSDME, resulting in diminished cell death. To validate these findings, we examined frontal cortical tissues from SIV-infected macaques, disclosing increased expression of GSDME and NINJ1 in cortical neurons, which was co-localized with caspase-3 detection in animals with neurological disease. Thus, HIV infection of the brain triggers the convergent activation of caspases-1 and -3, which results in GSDME-mediated neuronal pyroptosis in persons with HAND. These findings demonstrate a novel mechanism by which a viral infection causes pyroptotic death in neurons while also offering new diagnostic and therapeutic strategies for HAND and other neurodegenerative disorders.
Keywords: HIV; caspase; neurodegeneration; neuroinflammation; neuron; pyroptosis.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors report no competing interests.
Figures

Comment in
-
Pore-forming neurons: a new paradigm of pyroptotic cell death in HIV-associated neurocognitive disorder.Brain. 2024 Feb 1;147(2):335-336. doi: 10.1093/brain/awad435. Brain. 2024. PMID: 38134311 Free PMC article.
References
-
- McKenzie BA, Dixit VM, Power C. Fiery cell death: Pyroptosis in the central nervous system. Trends Neurosci. 2020;43:55–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

