Interferon-γ controls aquaporin 4-specific Th17 and B cells in neuromyelitis optica spectrum disorder
- PMID: 37931066
- PMCID: PMC10994540
- DOI: 10.1093/brain/awad373
Interferon-γ controls aquaporin 4-specific Th17 and B cells in neuromyelitis optica spectrum disorder
Abstract
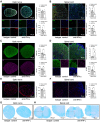
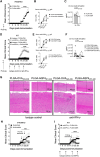
Neuromyelitis optica spectrum disorder (NMOSD) is a CNS autoimmune inflammatory disease mediated by T helper 17 (Th17) and antibody responses to the water channel protein, aquaporin 4 (AQP4), and associated with astrocytopathy, demyelination and axonal loss. Knowledge about disease pathogenesis is limited and the search for new therapies impeded by the absence of a reliable animal model. In our work, we determined that NMOSD is characterized by decreased IFN-γ receptor signalling and that IFN-γ depletion in AQP4201-220-immunized C57BL/6 mice results in severe clinical disease resembling human NMOSD. Pathologically, the disease causes autoimmune astrocytic and CNS injury secondary to cellular and humoral inflammation. Immunologically, the absence of IFN-γ allows for increased expression of IL-6 in B cells and activation of Th17 cells, and generation of a robust autoimmune inflammatory response. Consistent with NMOSD, the experimental disease is exacerbated by administration of IFN-β, whereas repletion of IFN-γ, as well as therapeutic targeting of IL-17A, IL-6R and B cells, ameliorates it. We also demonstrate that immune tolerization with AQP4201-220-coupled poly(lactic-co-glycolic acid) nanoparticles could both prevent and effectively treat the disease. Our findings enhance the understanding of NMOSD pathogenesis and provide a platform for the development of immune tolerance-based therapies, avoiding the limitations of the current immunosuppressive therapies.
Keywords: animal model; aquaporin 4; immune tolerance; interferon-γ; neuromyelitis optica spectrum disorder.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
S.D.M. is an academic co-founder, scientific advisory board member, paid consultant and grantee of COUR Pharmaceuticals and a consultant for NextCure and Takeda Pharmaceuticals. J.R.P. is an employee of COUR Pharmaceuticals. R.B. has received honoraria from Biogen, Teva Pharmaceuticals, Sanofi and Alexion. B.P. is a member of the scientific advisory board of Inflectis Bioscience. The other authors report no competing interests.
Figures






References
-
- Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, Wildemann B. Neuromyelitis optica. Nat Rev Dis Primers. 2020;6:85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

