Loss of p73 Expression Contributes to Chronic Obstructive Pulmonary Disease
- PMID: 37931077
- PMCID: PMC10806417
- DOI: 10.1164/rccm.202303-0503OC
Loss of p73 Expression Contributes to Chronic Obstructive Pulmonary Disease
Abstract
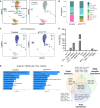
Rationale: Multiciliated cell (MCC) loss and/or dysfunction is common in the small airways of patients with chronic obstructive pulmonary disease (COPD), but it is unclear if this contributes to COPD lung pathology. Objectives: To determine if loss of p73 causes a COPD-like phenotype in mice and explore whether smoking or COPD impact p73 expression. Methods: p73floxE7-E9 mice were crossed with Shh-Cre mice to generate mice lacking MCCs in the airway epithelium. The resulting p73Δairway mice were analyzed using electron microscopy, flow cytometry, morphometry, forced oscillation technique, and single-cell RNA sequencing. Furthermore, the effects of cigarette smoke on p73 transcript and protein expression were examined using in vitro and in vivo models and in studies including airway epithelium from smokers and patients with COPD. Measurements and Main Results: Loss of functional p73 in the respiratory epithelium resulted in a near-complete absence of MCCs in p73Δairway mice. In adulthood, these mice spontaneously developed neutrophilic inflammation and emphysema-like lung remodeling and had progressive loss of secretory cells. Exposure of normal airway epithelium cells to cigarette smoke rapidly and durably suppressed p73 expression in vitro and in vivo. Furthermore, tumor protein 73 mRNA expression was reduced in the airways of current smokers (n = 82) compared with former smokers (n = 69), and p73-expressing MCCs were reduced in the small airways of patients with COPD (n = 11) compared with control subjects without COPD (n = 12). Conclusions: Loss of functional p73 in murine airway epithelium results in the absence of MCCs and promotes COPD-like lung pathology. In smokers and patients with COPD, loss of p73 may contribute to MCC loss or dysfunction.
Keywords: COPD; cigarette smoke; ciliated cell; p73.
Figures





Comment in
-
Are Airway Epithelial p73 Levels a Therapeutic Target to Treat Chronic Obstructive Pulmonary Disease?Am J Respir Crit Care Med. 2024 Jan 15;209(2):123-124. doi: 10.1164/rccm.202311-2073ED. Am J Respir Crit Care Med. 2024. PMID: 38051107 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

