Catalytic Mechanism of Collagen Hydrolysis by Zinc(II)-Dependent Matrix Metalloproteinase-1
- PMID: 37931179
- PMCID: PMC10659029
- DOI: 10.1021/acs.jpcb.3c04293
Catalytic Mechanism of Collagen Hydrolysis by Zinc(II)-Dependent Matrix Metalloproteinase-1
Abstract
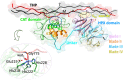
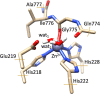
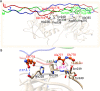
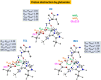
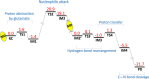
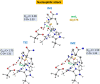
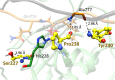
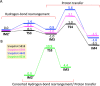
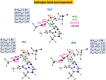
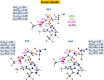
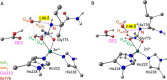
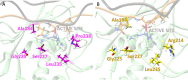
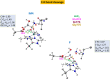
Human matrix metalloproteinase-1 (MMP-1) is a zinc(II)-dependent enzyme that catalyzes collagenolysis. Despite the availability of extensive experimental data, the mechanism of MMP-1-catalyzed collagenolysis remains poorly understood due to the lack of experimental structure of a catalytically productive enzyme-substrate complex of MMP-1. In this study, we apply molecular dynamics and combined quantum mechanics/molecular mechanics to reveal the reaction mechanism of MMP-1 based on a computationally modeled structure of the catalytically competent complex of MMP-1 that contains a large triple-helical peptide substrate. Our proposed mechanism involves the participation of an auxiliary (second) water molecule (wat2) in addition to the zinc(II)-coordinated water (wat1). The reaction initiates through a proton transfer to Glu219, followed by a nucleophilic attack by a zinc(II)-coordinated hydroxide anion nucleophile at the carbonyl carbon of the scissile bond, leading to the formation of a tetrahedral intermediate (IM2). The process continues with a hydrogen-bond rearrangement to facilitate proton transfer from wat2 to the amide nitrogen of the scissile bond and, finally, C-N bond cleavage. The calculations indicate that the rate-determining step is the water-mediated nucleophilic attack with an activation energy barrier of 22.3 kcal/mol. Furthermore, the calculations show that the hydrogen-bond rearrangement/proton-transfer step can proceed in a consecutive or concerted manner, depending on the conformation of the tetrahedral intermediate, with the consecutive mechanism being energetically preferable. Overall, the study reveals the crucial role of a second water molecule and the dynamics for effective MMP-1-catalyzed collagenolysis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Wezynfeld N. E.; Frączyk T.; Bal W. Metal Assisted Peptide Bond Hydrolysis: Chemistry, Biotechnology and Toxicological Implications. Coord. Chem. Rev. 2016, 327–328, 166–187. 10.1016/j.ccr.2016.02.009. - DOI
-
- Grant K.; Kassai M. Major Advances in the Hydrolysis of Peptides and Proteins by Metal Ions and Complexes. Curr. Org. Chem. 2006, 10 (9), 1035–1049. 10.2174/138527206777435535. - DOI
-
- Borel J. P.; Monboisse J. C.. Collagenolysis. Handbook Methods For Oxygen Radical Research, 1st ed.; CRC Press, 1985; p 6.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

