Efficacy and safety of remibrutinib, a selective potent oral BTK inhibitor, in Sjögren's syndrome: results from a randomised, double-blind, placebo-controlled phase 2 trial
- PMID: 37932009
- PMCID: PMC10894844
- DOI: 10.1136/ard-2023-224691
Efficacy and safety of remibrutinib, a selective potent oral BTK inhibitor, in Sjögren's syndrome: results from a randomised, double-blind, placebo-controlled phase 2 trial
Abstract
Objectives: To evaluate the safety and efficacy of remibrutinib in patients with moderate-to-severe Sjögren's syndrome (SjS) in a phase 2 randomised, double-blind trial (NCT04035668; LOUiSSE (LOU064 in Sjögren's Syndrome) study).
Methods: Eligible patients fulfilling 2016 American College of Rheumatology/European League Against Rheumatism (EULAR) criteria for SjS, positive for anti-Ro/Sjögren's syndrome-related antigen A antibodies, with moderate-to-severe disease activity (EULAR Sjögren's Syndrome Disease Activity Index (ESSDAI) (based on weighted score) ≥ 5, EULAR Sjögren's Syndrome Patient Reported Index (ESSPRI) ≥ 5) received remibrutinib (100 mg) either one or two times a day, or placebo for the 24-week study treatment period. The primary endpoint was change from baseline in ESSDAI at week 24. Key secondary endpoints included change from baseline in ESSDAI over time, change from baseline in ESSPRI over time and safety of remibrutinib in SjS. Key exploratory endpoints included changes to the salivary flow rate, soluble biomarkers, blood transcriptomic and serum proteomic profiles.
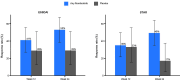
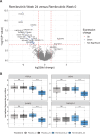
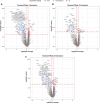
Results: Remibrutinib significantly improved ESSDAI score in patients with SjS over 24 weeks compared with placebo (ΔESSDAI -2.86, p=0.003). No treatment effect was observed in ESSPRI score (ΔESSPRI 0.17, p=0.663). There was a trend towards improvement of unstimulated salivary flow with remibrutinib compared with placebo over 24 weeks. Remibrutinib had a favourable safety profile in patients with SjS over 24 weeks. Remibrutinib induced significant changes in gene expression in blood, and serum protein abundance compared with placebo.
Conclusions: These data show preliminary efficacy and favourable safety of remibrutinib in a phase 2 trial for SjS.
Keywords: Patient Reported Outcome Measures; Sjogren's Syndrome; Therapeutics.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: T. Dörner declares editorial support from Novartis Pharma AG. In the past 36 months, he declares grants or contracts, and consultancy fees or honoraria for scientific advice from Eli Lilly, Sanofi, Novartis and Janssen, and he has participated on data safety monitoring boards for Novartis, Roche/Genentech and Boston Pharmaceuticals. M. Kaul is an employee of Novartis Pharma AG and retains Novartis stock and declares consultancy fees from Novartis Institutes for BioMedical Research. A. Szántó declares grant support for clinical study from Novartis, and support for attending meetings from Abbvie and CSL-Behring. J.C. Tseng declares medical writing support from Novartis. A. Papas declares institutional grant from Novartis for clinical study, and personal fees for participation on scientific advisory board from Novartis. I. Pylvaenaeinen is a full-time employee of Novartis Pharma AG and retains Novartis stock. M. Hanser is a full-time employee of Novartis Pharma AG. N. Abdallah is a full-time employee of Novartis Pharma AG. A. Grioni is a full-time employee of Novartis Pharma AG. A. Costa is a full-time employee of Novartis Pharma AG. E. Ferrero is a full-time employee of Novartis Pharma AG and retains Novartis stock. P. Gergely is a full-time employee of Novartis Pharma AG and retains Novartis stock. R. Hillenbrand is a full-time employee of Novartis Pharma AG and retains Novartis stock. A. Avrameas is a full-time employee of Novartis Pharma AG. B. Cenni is a full-time employee of Novartis Pharma AG, retains Novartis stock and declares patents with Novartis Institutes for BioMedical Research. R. Siegel is a full-time employee of Novartis Pharma AG and retains Novartis stock.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

