Abatacept and non-melanoma skin cancer in patients with rheumatoid arthritis: a comprehensive evaluation of randomised controlled trials and observational studies
- PMID: 37932010
- PMCID: PMC10850629
- DOI: 10.1136/ard-2023-224356
Abatacept and non-melanoma skin cancer in patients with rheumatoid arthritis: a comprehensive evaluation of randomised controlled trials and observational studies
Abstract
Objectives: This study aims to evaluate non-melanoma skin cancer (NMSC) risk associated with abatacept treatment for rheumatoid arthritis (RA).
Methods: This evaluation included 16 abatacept RA clinical trials and 6 observational studies. NMSC incidence rates (IRs)/1000 patient-years (p-y) of exposure were compared between patients treated with abatacept versus placebo, conventional synthetic (cs) disease-modifying antirheumatic drugs (DMARDs) and other biological/targeted synthetic (b/ts)DMARDs. For observational studies, a random-effects model was used to pool rate ratios (RRs).
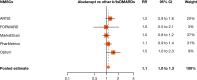
Results: ~49 000 patients receiving abatacept were analysed from clinical trials (~7000) and observational studies (~42 000). In randomised trials (n=4138; median abatacept exposure, 12 (range 2-30) months), NMSC IRs (95% CIs) were not significantly different for abatacept (6.0 (3.3 to 10.0)) and placebo (4.0 (1.3 to 9.3)) and remained stable throughout the long-term, open-label period (median cumulative exposure, 28 (range 2-130 months); 21 335 p-y of exposure (7044 patients over 3 years)). For registry databases, NMSC IRs/1000 p-y were 5-12 (abatacept), 1.6-10 (csDMARDs) and 3-8 (other b/tsDMARDs). Claims database IRs were 19-22 (abatacept), 15-18 (csDMARDs) and 14-17 (other b/tsDMARDs). Pooled RRs (95% CIs) from observational studies for NMSC in patients receiving abatacept were 1.84 (1.00 to 3.37) vs csDMARDs and 1.11 (0.98 to 1.26) vs other b/tsDMARDs.
Conclusions: Consistent with the warnings and precautions of the abatacept label, this analysis suggests a potential increase in NMSC risk with abatacept use compared with csDMARDs. No significant increase was observed compared with b/tsDMARDs, but the lower limit of the 95% CI was close to unity.
Keywords: abatacept; arthritis, rheumatoid; biological therapy; epidemiology.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: TS was an employee of and shareholder in Bristol Myers Squibb (at the time of the analysis; former employee at present). LD, VK, AD and MAM are employees of and shareholders in Bristol Myers Squibb. SS reports advisory board involvement, speaker fees and grant/research support from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, and Novartis. KM reports grant/research support from Rheumatology Research Foundation. MH is an employee of the University of Maryland School of Medicine (full time) and US Department of Veterans Affairs (part time); reports consultancy fees and advisory board involvement from Bristol Myers Squibb, Eli Lilly, Kolon TissueGene, Inc, Novartis, Pfizer, Samumed and Theralogix; is a member of the Data Safety Monitoring Committee for Galapagos, Roche, and IQVIA; reports royalties from Elsevier and UpToDate; reports stock ownership in BriOri Biotech and Theralogix; and is president of Rheumcon. MB reports consultancy fees from Novartis. JA reports grant/research support from AbbVie, Bristol Myers Squibb, Eli Lilly, Galapagos, Merck, Pfizer, Roche, Samsung Bioepis, Sanofi and UCB for ARTIS. AS reports speaker fees from AbbVie, Bristol Myers Squibb, Celltrion, Lilly, Merck, Pfizer and Roche.
Figures

Similar articles
-
Malignancy outcomes in patients with rheumatoid arthritis treated with abatacept and other disease-modifying antirheumatic drugs: Results from a 10-year international post-marketing study.Semin Arthritis Rheum. 2024 Feb;64:152240. doi: 10.1016/j.semarthrit.2023.152240. Epub 2023 Jun 30. Semin Arthritis Rheum. 2024. PMID: 37500379
-
Infection outcomes in patients with rheumatoid arthritis treated with abatacept and other disease-modifying antirheumatic drugs: Results from a 10-year international post-marketing study.Semin Arthritis Rheum. 2024 Feb;64:152313. doi: 10.1016/j.semarthrit.2023.152313. Epub 2023 Nov 10. Semin Arthritis Rheum. 2024. PMID: 38044241
-
Comparative risk of malignancies and infections in patients with rheumatoid arthritis initiating abatacept versus other biologics: a multi-database real-world study.Arthritis Res Ther. 2019 Nov 8;21(1):228. doi: 10.1186/s13075-019-1992-x. Arthritis Res Ther. 2019. PMID: 31703717 Free PMC article. Clinical Trial.
-
Analysis of efficacy and safety of abatacept for rheumatoid arthritis: systematic review and meta-analysis.Clin Exp Rheumatol. 2023 Sep;41(9):1882-1900. doi: 10.55563/clinexprheumatol/2xjg0d. Epub 2023 Mar 7. Clin Exp Rheumatol. 2023. PMID: 36912326
-
Most Appropriate Conventional Disease-Modifying Antirheumatic Drug to Combine With Different Advanced Therapies in Rheumatoid Arthritis: A Systematic Literature Review With Meta-Analysis.Arthritis Care Res (Hoboken). 2021 Jun;73(6):873-884. doi: 10.1002/acr.24195. Arthritis Care Res (Hoboken). 2021. PMID: 32216091
Cited by
-
Belatacept and non-melanoma skin cancer risk in kidney transplant recipients: a narrative review from a mechanistic and clinical perspective.BMC Nephrol. 2025 Aug 23;26(1):487. doi: 10.1186/s12882-025-04373-z. BMC Nephrol. 2025. PMID: 40849621 Free PMC article. Review.
-
Abatacept and the risk of malignancy: a meta-analysis across disease indications.Rheumatology (Oxford). 2025 Jun 1;64(6):3280-3287. doi: 10.1093/rheumatology/keaf114. Rheumatology (Oxford). 2025. PMID: 39992258 Free PMC article.
-
Solid Cancers and Rheumatoid Arthritis.Cancers (Basel). 2023 Nov 16;15(22):5441. doi: 10.3390/cancers15225441. Cancers (Basel). 2023. PMID: 38001700 Free PMC article.
-
Beliefs, preferences, and informational needs of patients with rheumatoid arthritis and concomitant cancer: a qualitative study.BMC Rheumatol. 2025 Jul 1;9(1):79. doi: 10.1186/s41927-025-00526-7. BMC Rheumatol. 2025. PMID: 40598674 Free PMC article.
References
-
- Mercer LK, Green AC, Galloway JB, et al. . The influence of anti-TNF therapy upon incidence of keratinocyte skin cancer in patients with rheumatoid arthritis: longitudinal results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2012;71:869–74. 10.1136/annrheumdis-2011-200622 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

