Aotus nancymaae model predicts human immune response to the placental malaria vaccine candidate VAR2CSA
- PMID: 37932470
- PMCID: PMC10689237
- DOI: 10.1038/s41684-023-01274-2
Aotus nancymaae model predicts human immune response to the placental malaria vaccine candidate VAR2CSA
Abstract
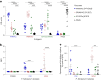
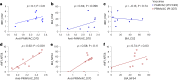
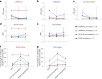
Placental malaria vaccines (PMVs) are being developed to prevent severe sequelae of placental malaria (PM) in pregnant women and their offspring. The leading candidate vaccine antigen VAR2CSA mediates parasite binding to placental receptor chondroitin sulfate A (CSA). Despite promising results in small animal studies, recent human trials of the first two PMV candidates (PAMVAC and PRIMVAC) generated limited cross-reactivity and cross-inhibitory activity to heterologous parasites. Here we immunized Aotus nancymaae monkeys with three PMV candidates (PAMVAC, PRIMVAC and ID1-ID2a_M1010) adjuvanted with Alhydrogel, and exploited the model to investigate boosting of functional vaccine responses during PM episodes as well as with nanoparticle antigens. PMV candidates induced high levels of antigen-specific IgG with significant cross-reactivity across PMV antigens by enzyme-linked immunosorbent assay. Conversely, PMV antibodies recognized native VAR2CSA and blocked CSA adhesion of only homologous parasites and not of heterologous parasites. PM episodes did not significantly boost VAR2CSA antibody levels or serum functional activity; nanoparticle and monomer antigens alike boosted serum reactivity but not functional activities. Overall, PMV candidates induced functional antibodies with limited heterologous activity in Aotus monkeys, similar to responses reported in humans. The Aotus model appears suitable for preclinical downselection of PMV candidates and assessment of antibody boosting by PM episodes.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
T.G.T. and A. Salanti are named on a patent owned by University of Copenhagen for the use of VAR2CSA as a malaria vaccine. M.A.N., A.F.S., C.M.J., A. Salanti and T.G.T. are named on a patent to use capsid particles in vaccine development. The other authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- 42387 and OPP1055855/Bill and Melinda Gates Foundation (Bill & Melinda Gates Foundation)
- ANR-16-CE11-0014-01/Agence Nationale de la Recherche (French National Research Agency)
- ANR-11-IDEX-0005-02/Agence Nationale de la Recherche (French National Research Agency)
- 202060457/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
LinkOut - more resources
Full Text Sources
Medical

