Plasma Aβ42/Aβ40 and phospho-tau217 concentration ratios increase the accuracy of amyloid PET classification in preclinical Alzheimer's disease
- PMID: 37932961
- PMCID: PMC10916957
- DOI: 10.1002/alz.13542
Plasma Aβ42/Aβ40 and phospho-tau217 concentration ratios increase the accuracy of amyloid PET classification in preclinical Alzheimer's disease
Abstract
Introduction: Incorporating blood-based Alzheimer's disease biomarkers such as tau and amyloid beta (Aβ) into screening algorithms may improve screening efficiency.
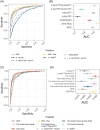
Methods: Plasma Aβ, phosphorylated tau (p-tau)181, and p-tau217 concentration levels from AHEAD 3-45 study participants were measured using mass spectrometry. Tau concentration ratios for each proteoform were calculated to normalize for inter-individual differences. Receiver operating characteristic (ROC) curve analysis was performed for each biomarker against amyloid positivity, defined by > 20 Centiloids. Mixture of experts analysis assessed the value of including tau concentration ratios into the existing predictive algorithm for amyloid positron emission tomography status.
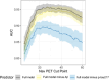
Results: The area under the receiver operating curve (AUC) was 0.87 for Aβ42/Aβ40, 0.74 for phosphorylated variant p-tau181 ratio (p-tau181/np-tau181), and 0.92 for phosphorylated variant p-tau217 ratio (p-tau217/np-tau217). The Plasma Predicted Centiloid (PPC), a predictive model including p-tau217/np-tau217, Aβ42/Aβ40, age, and apolipoprotein E improved AUC to 0.95.
Discussion: Including plasma p-tau217/np-tau217 along with Aβ42/Aβ40 in predictive algorithms may streamline screening preclinical individuals into anti-amyloid clinical trials.
Clinicaltrials: gov Identifier: NCT04468659 HIGHLIGHTS: The addition of plasma phosphorylated variant p-tau217 ratio (p-tau217/np-tau217) significantly improved plasma biomarker algorithms for identifying preclinical amyloid positron emission tomography positivity. Prediction performance at higher NAV Centiloid levels was improved with p-tau217/np-tau217. All models generated for this study are incorporated into the Plasma Predicted Centiloid (PPC) app for public use.
Keywords: blood-based biomarkers; mass spectrometry; plasma; tau.
© 2023 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Sara Abdel‐Latif, Jennifer Ngolab, and Keith A. Johnson have nothing to disclose. Oliver Langford is an employee of the Keck School of Medicine of USC. Matthew R. Meyer, Traci Wente‐Roth, Kristopher M. Kirmess, Tim West, Kevin E. Yarasheski, and Joel B. Braunstein are paid employees of C2N Diagnostics. Kevin E. Yarasheski has research grants from the NIH, BrightFocus Foundation, GHR Foundation, and Alzheimer's Drug Discovery Foundation. Pallavi Sachdev and Michael Irizarry are paid employees of Eisai. Robert A. Rissman, Rema Raman, Michael C. Donohue, and Charisse N. Winston have grants from the NIH. Rema Raman has received grants from Eisai, Eli Lilly, the Alzheimer's Association, and the American Heart Association. Michael C. Donohue serves as a consultant for Roche and has a spouse working at Janssen. Gustavo Jimenez‐Maggiora has received support from C2N Diagnostics, Eisai Co, Ltd, and the NIH. Michael S. Rafii serves as a consultant for AC Immune, Ionis, Keystone Bio, and Positrigo. Paul S. Aisen has research grants from Eisai, NIH, Lilly, the Alzheimer's Association, and Janssen and serves as a consultant for Merck, Bristol Myers Squibb, Switch Therapeutics, Roche, Arrowhead, ImmunoBrain, Checkpoint, Biogen, Abbvie, Genetech, and NewAmsterdam Pharma. Reisa A. Sperling has grants from the NIH, the Alzheimer's Association, the GHR Foundation, Eli Lilly, and Eisai, and has consulted for AC Immune, Acumen, Alector, Alnylam, Cytox, Genentech, Janssen, JOMDD, Nervgen, Neuraly, Neurocentria, Oligomerix, Prothena, Renew, Shionogi, Vigil Neuroscience, Ionis, Vaxxinity, and Bristol Myers Squibb. Author disclosures are available in the supporting information.
Figures

References
-
- Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS‐ADRDA criteria. Lancet Neurol. 2007;6:734‐746. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R44 AG059489/AG/NIA NIH HHS/United States
- R01 AG073979/AG/NIA NIH HHS/United States
- R37 AG018440/AG/NIA NIH HHS/United States
- R01 AG053798/AG/NIA NIH HHS/United States
- U19 AG078109/AG/NIA NIH HHS/United States
- R01 AG058533/AG/NIA NIH HHS/United States
- K99 AG070390/AG/NIA NIH HHS/United States
- R01 AG061848/AG/NIA NIH HHS/United States
- R01 AG050595/AG/NIA NIH HHS/United States
- R01 AG054029/AG/NIA NIH HHS/United States
- R01 AG058252/AG/NIA NIH HHS/United States
- RF1 AG073979/AG/NIA NIH HHS/United States
- U24 AG057437/AG/NIA NIH HHS/United States
- R25 NS117367/NS/NINDS NIH HHS/United States
- R56 AG058533/AG/NIA NIH HHS/United States
- R01 AG018440/AG/NIA NIH HHS/United States
- P30 AG066530/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

