Programmable kernel structures of atomically precise metal nanoclusters for tailoring catalytic properties
- PMID: 37933377
- PMCID: PMC10624382
- DOI: 10.1002/EXP.20220005
Programmable kernel structures of atomically precise metal nanoclusters for tailoring catalytic properties
Abstract
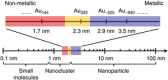
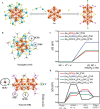
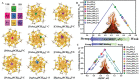
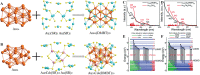
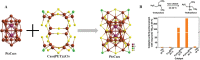
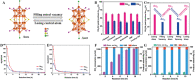
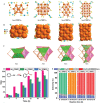
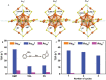
The unclear structures and polydispersity of metal nanoparticles (NPs) seriously hamper the identification of the active sites and the construction of structure-reactivity relationships. Fortunately, ligand-protected metal nanoclusters (NCs) with atomically precise structures and monodispersity have become an ideal candidate for understanding the well-defined correlations between structure and catalytic property at an atomic level. The programmable kernel structures of atomically precise metal NCs provide a fantastic chance to modulate their size, shape, atomic arrangement, and electron state by the precise modulating of the number, type, and location of metal atoms. Thus, the special focus of this review highlights the most recent process in tailoring the catalytic activity and selectivity over metal NCs by precisely controlling their kernel structures. This review is expected to shed light on the in-depth understanding of metal NCs' kernel structures and reactivity relationships.
Keywords: atomically precise nanocluster; catalysis; kernel structure; structure‐reactivity relationships.
© 2023 The Authors. Exploration published by Henan University and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Gao C., Lyu F., Yin Y., Chem. Rev. 2021, 121, 834. - PubMed
-
- Jin R., Nanotechnol. Rev. 2012, 1, 31.
-
- Chen T.‐T., Wang R., Li L.‐K., Li Z.‐J., Zang S.‐Q., J. Energy Chem. 2020, 44, 90.
Publication types
LinkOut - more resources
Full Text Sources
