Efficacy of spironolactone as adjunctive therapy to sodium valproate in bipolar-I disorder: A double-blind, randomized, placebo-controlled clinical trial
- PMID: 37933420
- PMCID: PMC10726882
- DOI: 10.1002/brb3.3313
Efficacy of spironolactone as adjunctive therapy to sodium valproate in bipolar-I disorder: A double-blind, randomized, placebo-controlled clinical trial
Abstract
Introduction: Treatment of mood and cognitive symptoms of patients with bipolar disorder is associated with many complications and is generally not associated with therapeutic satisfaction. In this clinical trial, we evaluated the effectiveness of spironolactone in controlling mood and cognitive symptoms, sleep quality, appetite, and body mass index in patients with bipolar disorder in manic episodes.
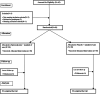
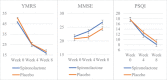
Methods: Sixty inpatients with bipolar disorder in manic episodes were treated with spironolactone/placebo in an 8-week randomized, double-blind, placebo-controlled clinical trial. They were evaluated using the Young Mania Rating Scale (YMRS), mini-mental state examination (MMSE), Pittsburgh sleep quality index, Simplified Nutritional Appetite Questionnaire, and body mass index in weeks 1, 4, and 8.
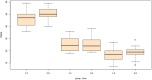
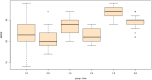
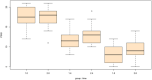
Results: For cognitive impairment (MMSE), there were significant interaction effects of group and time at week 8 (B = -1.60, SE = 0.69, t = -2.33, p = .021) such that individuals in the spironolactone group experienced more improvement in their cognitive performance. For manic symptoms (YMRS), there were no significant interaction effects of group and time at week 8 (B = -2.53, SE = 1.46, t = -1.73, p = .085).
Conclusions: Considering the promising findings in this clinical trial, further study of spironolactone as adjunctive therapy in bipolar disorder in manic episodes with larger sample sizes, multicenter settings, and longer follow-ups are recommended.
Keywords: bipolar disorder; cognition; hypothalamus-pituitary-adrenal axis; spironolactone.
© 2023 The Authors. Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Celecoxib adjunctive therapy for acute bipolar mania: a randomized, double-blind, placebo-controlled trial.Bipolar Disord. 2015 Sep;17(6):606-14. doi: 10.1111/bdi.12324. Epub 2015 Aug 20. Bipolar Disord. 2015. PMID: 26291962 Clinical Trial.
-
[Antipsychotics in bipolar disorders].Encephale. 2004 Sep-Oct;30(5):417-24. doi: 10.1016/s0013-7006(04)95456-5. Encephale. 2004. PMID: 15627046 Review. French.
-
Short-Term Effects of Folate Supplementation in Combination With Vitamin B6 for Treating Acute Manic Episodes in Bipolar I Disorder: A Randomized Controlled Trial.Brain Behav. 2025 Apr;15(4):e70432. doi: 10.1002/brb3.70432. Brain Behav. 2025. PMID: 40200764 Free PMC article. Clinical Trial.
-
Olanzapine-divalproex combination versus divalproex monotherapy in the treatment of bipolar mixed episodes: a double-blind, placebo-controlled study.J Clin Psychiatry. 2009 Nov;70(11):1540-7. doi: 10.4088/JCP.08m04895yel. Epub 2009 Sep 22. J Clin Psychiatry. 2009. PMID: 19778495 Clinical Trial.
-
Asenapine: a review of its use in the management of mania in adults with bipolar I disorder.CNS Drugs. 2011 Mar;25(3):251-67. doi: 10.2165/11206700-000000000-00000. CNS Drugs. 2011. PMID: 21323396 Review.
Cited by
-
Skin-brain dialogue in auto-inflammatory diseases: A new route to biomarkers?Brain Behav Immun Health. 2024 Nov 9;42:100906. doi: 10.1016/j.bbih.2024.100906. eCollection 2024 Dec. Brain Behav Immun Health. 2024. PMID: 39624485 Free PMC article.
-
Mineralocorticoid Receptor Antagonists and Cognitive Outcomes in Cardiovascular Disease and Beyond: A Systematic Review.J Pers Med. 2025 Jan 30;15(2):57. doi: 10.3390/jpm15020057. J Pers Med. 2025. PMID: 39997334 Free PMC article. Review.
-
A second act for spironolactone: cognitive benefits in renal dysfunction - a critical review.Metab Brain Dis. 2025 Apr 29;40(5):194. doi: 10.1007/s11011-025-01623-9. Metab Brain Dis. 2025. PMID: 40299184 Review.
References
-
- Akesode, A. , Hendler, N. , & Kowarski, A. A. (1976). A 24‐hour monitoring of the integrated plasma concentration of aldosterone and cortisol in manic patients. Psychoneuroendocrinology, 1(4), 419–426. 10.1016/0306-4530(76)90009-3 - DOI
-
- Alheira, F. V. , & Brasil, M. A. A. (2005). The role of glucocorticoids in the modulation of mood symptoms: A review. Revista de Psiquiatria do Rio Grande do Sul, 27, 177–186. 10.1590/S0101-81082005000200008 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

