Differing structures and dynamics of two photolesions portray verification differences by the human XPD helicase
- PMID: 37933861
- PMCID: PMC10711554
- DOI: 10.1093/nar/gkad974
Differing structures and dynamics of two photolesions portray verification differences by the human XPD helicase
Abstract
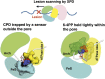
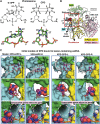
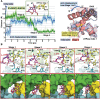
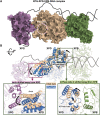
Ultraviolet light generates cyclobutane pyrimidine dimer (CPD) and pyrimidine 6-4 pyrimidone (6-4PP) photoproducts that cause skin malignancies if not repaired by nucleotide excision repair (NER). While the faster repair of the more distorting 6-4PPs is attributed mainly to more efficient recognition by XPC, the XPD lesion verification helicase may play a role, as it directly scans the damaged DNA strand. With extensive molecular dynamics simulations of XPD-bound single-strand DNA containing each lesion outside the entry pore of XPD, we elucidate strikingly different verification processes for these two lesions that have very different topologies. The open book-like CPD thymines are sterically blocked from pore entry and preferably entrapped by sensors that are outside the pore; however, the near-perpendicular 6-4PP thymines can enter, accompanied by a displacement of the Arch domain toward the lesion, which is thereby tightly accommodated within the pore. This trapped 6-4PP may inhibit XPD helicase activity to foster lesion verification by locking the Arch to other domains. Furthermore, the movement of the Arch domain, only in the case of 6-4PP, may trigger signaling to the XPG nuclease for subsequent lesion incision by fostering direct contact between the Arch domain and XPG, and thereby facilitating repair of 6-4PP.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Cadet J., Douki T.. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem. Photobiol. Sci. 2018; 17:1816–1841. - PubMed
-
- Sancar A. Mechanisms of DNA repair by photolyase and excision nuclease (Nobel Lecture). Angew. Chem. Int. Ed Engl. 2016; 55:8502–8527. - PubMed
-
- Hoeijmakers J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009; 361:1475–1485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

