Disruption of SUV39H1-Mediated H3K9 Methylation Sustains CAR T-cell Function
- PMID: 37934007
- PMCID: PMC10880746
- DOI: 10.1158/2159-8290.CD-22-1319
Disruption of SUV39H1-Mediated H3K9 Methylation Sustains CAR T-cell Function
Abstract
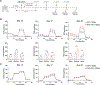
Suboptimal functional persistence limits the efficacy of adoptive T-cell therapies. CD28-based chimeric antigen receptors (CAR) impart potent effector function to T cells but with a limited lifespan. We show here that the genetic disruption of SUV39H1, which encodes a histone-3, lysine-9 methyl-transferase, enhances the early expansion, long-term persistence, and overall antitumor efficacy of human CAR T cells in leukemia and prostate cancer models. Persisting SUV39H1-edited CAR T cells demonstrate improved expansion and tumor rejection upon multiple rechallenges. Transcriptional and genome accessibility profiling of repeatedly challenged CAR T cells shows improved expression and accessibility of memory transcription factors in SUV39H1-edited CAR T cells. SUV39H1 editing also reduces expression of inhibitory receptors and limits exhaustion in CAR T cells that have undergone multiple rechallenges. Our findings thus demonstrate the potential of epigenetic programming of CAR T cells to balance their function and persistence for improved adoptive cell therapies.
Significance: T cells engineered with CD28-based CARs possess robust effector function and antigen sensitivity but are hampered by limited persistence, which may result in tumor relapse. We report an epigenetic strategy involving disruption of the SUV39H1-mediated histone-silencing program that promotes the functional persistence of CD28-based CAR T cells. See related article by López-Cobo et al., p. 120. This article is featured in Selected Articles from This Issue, p. 5.
©2023 American Association for Cancer Research.
Conflict of interest statement
Author’s Disclosures
M.S. is a shareholder in Mnemo Therapeutics which holds license to patents on SUV39H1 inactivation. T.G. is an employee of Mnemo Therapeutics. All other authors declare no conflict of interest.
Authors’ Disclosure
The authors declare no competing interests.
Figures



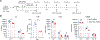


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

