Contractility measurements for cardiotoxicity screening with ventricular myocardial slices of pigs
- PMID: 37934066
- PMCID: PMC10651213
- DOI: 10.1093/cvr/cvad141
Contractility measurements for cardiotoxicity screening with ventricular myocardial slices of pigs
Erratum in
-
Correction to: Contractility measurements for cardiotoxicity screening with ventricular myocardial slices of pigs.Cardiovasc Res. 2024 Mar 30;120(4):434. doi: 10.1093/cvr/cvad187. Cardiovasc Res. 2024. PMID: 38118109 Free PMC article. No abstract available.
Abstract
Aims: Cardiotoxicity is one major reason why drugs do not enter or are withdrawn from the market. Thus, approaches are required to predict cardiotoxicity with high specificity and sensitivity. Ideally, such methods should be performed within intact cardiac tissue with high relevance for humans and detect acute and chronic side effects on electrophysiological behaviour, contractility, and tissue structure in an unbiased manner. Herein, we evaluate healthy pig myocardial slices and biomimetic cultivation setups (BMCS) as a new cardiotoxicity screening approach.
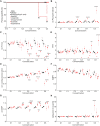
Methods and results: Pig left ventricular samples were cut into slices and spanned into BMCS with continuous electrical pacing and online force recording. Automated stimulation protocols were established to determine the force-frequency relationship (FFR), frequency dependence of contraction duration, effective refractory period (ERP), and pacing threshold. Slices generated 1.3 ± 0.14 mN/mm2 force at 0.5 Hz electrical pacing and showed a positive FFR and a shortening of contraction duration with increasing pacing rates. Approximately 62% of slices were able to contract for at least 6 days while showing stable ERP, contraction duration-frequency relationship, and preserved cardiac structure confirmed by confocal imaging and X-ray diffraction analysis. We used specific blockers of the most important cardiac ion channels to determine which analysis parameters are influenced. To validate our approach, we tested five drug candidates selected from the Comprehensive in vitro Proarrhythmia Assay list as well as acetylsalicylic acid and DMSO as controls in a blinded manner in three independent laboratories. We were able to detect all arrhythmic drugs and their respective mode of action on cardiac tissue including inhibition of Na+, Ca2+, and hERG channels as well as Na+/Ca2+ exchanger.
Conclusion: We systematically evaluate this approach for cardiotoxicity screening, which is of high relevance for humans and can be upscaled to medium-throughput screening. Thus, our approach will improve the predictive value and efficiency of preclinical cardiotoxicity screening.
Keywords: Cardiac arrhythmia; Cardiotoxicity; Drug-induced long QT syndrome; Screening; Ventricular slices.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: A.D. and Th.S. are shareholders of InVitroSys GmbH. There are no other remaining competing interests.
Figures



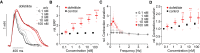


References
-
- Li X, Zhang R, Zhao B, Lossin C, Cao Z. Cardiotoxicity screening: a review of rapid-throughput in vitro approaches. Arch Toxicol 2016;90:1803–1816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

