A 20-year Follow-up of the International Early Lung Cancer Action Program (I-ELCAP)
- PMID: 37934099
- PMCID: PMC10698500
- DOI: 10.1148/radiol.231988
A 20-year Follow-up of the International Early Lung Cancer Action Program (I-ELCAP)
Abstract
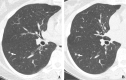
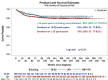
Background The low-dose CT (≤3 mGy) screening report of 1000 Early Lung Cancer Action Program (ELCAP) participants in 1999 led to the International ELCAP (I-ELCAP) collaboration, which enrolled 31 567 participants in annual low-dose CT screening between 1992 and 2005. In 2006, I-ELCAP investigators reported the 10-year lung cancer-specific survival of 80% for 484 participants diagnosed with a first primary lung cancer through annual screening, with a high frequency of clinical stage I lung cancer (85%). Purpose To update the cure rate by determining the 20-year lung cancer-specific survival of participants diagnosed with first primary lung cancer through annual low-dose CT screening in the expanded I-ELCAP cohort. Materials and Methods For participants enrolled in the HIPAA-compliant prospective I-ELCAP cohort between 1992 and 2022 and observed until December 30, 2022, Kaplan-Meier survival analysis was used to determine the 10- and 20-year lung cancer-specific survival of participants diagnosed with first primary lung cancer through annual low-dose CT screening. Eligible participants were aged at least 40 years and had current or former cigarette use or had never smoked but had been exposed to secondhand tobacco smoke. Results Among 89 404 I-ELCAP participants, 1257 (1.4%) were diagnosed with a first primary lung cancer (684 male, 573 female; median age, 66 years; IQR, 61-72), with a median smoking history of 43.0 pack-years (IQR, 29.0-60.0). Median follow-up duration was 105 months (IQR, 41-182). The frequency of clinical stage I at pretreatment CT was 81% (1017 of 1257). The 10-year lung cancer-specific survival of 1257 participants was 81% (95% CI: 79, 84) and the 20-year lung cancer-specific survival was 81% (95% CI: 78, 83), and it was 95% (95% CI: 91, 98) for 181 participants with pathologic T1aN0M0 lung cancer. Conclusion The 10-year lung cancer-specific survival of 80% reported in 2006 for I-ELCAP participants enrolled in annual low-dose CT screening and diagnosed with a first primary lung cancer has persisted, as shown by the updated 20-year lung cancer-specific survival for the expanded I-ELCAP cohort. © RSNA, 2023 See also the editorials by Grenier and by Sequist and Olazagasti in this issue.
Conflict of interest statement
Figures


Comment in
-
Cure Rate of Lung Cancer Diagnosed at Annual CT Screening.Radiology. 2023 Nov;309(2):e232698. doi: 10.1148/radiol.232698. Radiology. 2023. PMID: 37934092 No abstract available.
-
Twenty-year Progress in Lung Cancer Screening: A Marathon, Not a Sprint.Radiology. 2023 Nov;309(2):e232850. doi: 10.1148/radiol.232850. Radiology. 2023. PMID: 37934096 Free PMC article. No abstract available.
References
-
- Henschke CI , McCauley DI , Yankelevitz DF , et al . Early Lung Cancer Action Project: overall design and findings from baseline screening . Lancet 1999. ; 354 ( 9173 ): 99 – 105 . - PubMed
-
- Henschke CI , Miettinen OS , Yankelevitz DF , Libby DM , Smith JP . Radiographic screening for cancer . Proposed paradigm for requisite research . Clin Imaging 1994. ; 18 ( 1 ): 16 – 20 . - PubMed
-
- Grady D . CAT scan process could cut deaths from lung cancer . New York Times , July 9, 1999. https://www.nytimes.com/1999/07/09/us/cat-scan-process-could-cut-deaths-.... Accessed October 23, 2023 .
-
- National Cancer Advisory Board . National Cancer Institute. Spiral Computed Tomography (CT) Scanning for Detection of Lung Cancer . In: National Cancer Advisory Board 111st Regular Meeting Minutes , September 23–24, 1999 Bethesda, Md : National Institutes of Health; , 1999. ; 18 . https://deainfo.nci.nih.gov/advisory/ncab/archive/111_0999/ncab0999.pdf. Accessed October 23, 2023 .
-
- Henschke CI , Yankelevitz DF , Smith JP , Miettinen OS ; ELCAP Group . Screening for lung cancer: the early lung cancer action approach . Lung Cancer 2002. ; 35 ( 2 ): 143 – 148 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

