Vascular dysfunction and the age-related decline in critical power
- PMID: 37934136
- PMCID: PMC10988715
- DOI: 10.1113/EP091571
Vascular dysfunction and the age-related decline in critical power
Abstract
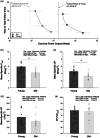
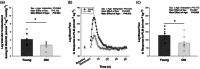
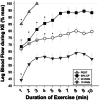
Ageing results in lower exercise tolerance, manifested as decreased critical power (CP). We examined whether the age-related decrease in CP occurs independently of changes in muscle mass and whether it is related to impaired vascular function. Ten older (63.1 ± 2.5 years) and 10 younger (24.4 ± 4.0 years) physically active volunteers participated. Physical activity was measured with accelerometry. Leg muscle mass was quantified with dual X-ray absorptiometry. The CP and maximum power during a graded exercise test (PGXT ) of single-leg knee-extension exercise were determined over the course of four visits. During a fifth visit, vascular function of the leg was assessed with passive leg movement (PLM) hyperaemia and leg blood flow and vascular conductance during knee-extension exercise at 10 W, 20 W, slightly below CP (90% CP) and PGXT . Despite not differing in leg lean mass (P = 0.901) and physical activity (e.g., steps per day, P = 0.735), older subjects had ∼30% lower mass-specific CP (old = 3.20 ± 0.94 W kg-1 vs. young = 4.60 ± 0.87 W kg-1 ; P < 0.001). The PLM-induced hyperaemia and leg blood flow and/or conductance were blunted in the old at 20 W, 90% CP and PGXT (P < 0.05). When normalized for leg muscle mass, CP was strongly correlated with PLM-induced hyperaemia (R2 = 0.52; P < 0.001) and vascular conductance during knee-extension exercise at 20 W (R2 = 0.34; P = 0.014) and 90% CP (R2 = 0.39; P = 0.004). In conclusion, the age-related decline in CP is not only an issue of muscle quantity, but also of impaired muscle quality that corresponds to impaired vascular function.
Keywords: ageing; endothelial function; exercise blood flow; exercise intolerance.
© 2023 The Authors. Experimental Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Broxterman, R. M. , Ade, C. J. , Wilcox, S. L. , Schlup, S. J. , Craig, J. C. , & Barstow, T. J. (2014). Influence of duty cycle on the power‐duration relationship: Observations and potential mechanisms. Respiratory Physiology & Neurobiology, 192, 102–111. - PubMed
-
- Broxterman, R. M. , Trinity, J. D. , Gifford, J. R. , Kwon, O. S. , Kithas, A. C. , Hydren, J. R. , Nelson, A. D. , Morgan, D. E. , Jessop, J. E. , Bledsoe, A. D. , & Richardson, R. S. (2017). Single passive leg movement assessment of vascular function: Contribution of nitric oxide. Journal of Applied Physiology (1985), 123(6), 1468–1476. - PMC - PubMed
-
- Collins, J. , Leach, O. , Dorff, A. , Linde, J. , Kofoed, J. , Sherman, M. , Proffit, M. , & Gifford, J. R. (2022). Critical power and work‐prime account for variability in endurance training adaptations not captured by V̇o(2max). Journal of Applied Physiology (1985), 133(4), 986–1000. - PubMed
-
- Craig, J. C. , Colburn, T. D. , Caldwell, J. T. , Hirai, D. M. , Tabuchi, A. , Baumfalk, D. R. , Behnke, B. J. , Ade, C. J. , Musch, T. I. , & Poole, D. C. (2019). Central and peripheral factors mechanistically linked to exercise intolerance in heart failure with reduced ejection fraction. American Journal of Physiology. Heart and Circulatory Physiology, 317(2), H434–H444. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
