Pharmacotherapy for Alcohol Use Disorder: A Systematic Review and Meta-Analysis
- PMID: 37934220
- PMCID: PMC10630900
- DOI: 10.1001/jama.2023.19761
Pharmacotherapy for Alcohol Use Disorder: A Systematic Review and Meta-Analysis
Erratum in
-
Error in Byline.JAMA. 2024 Oct 2;332(16):1397. doi: 10.1001/jama.2024.11331. Online ahead of print. JAMA. 2024. PMID: 39356516 Free PMC article. No abstract available.
Abstract
Importance: Alcohol use disorder affects more than 28.3 million people in the United States and is associated with increased rates of morbidity and mortality.
Objective: To compare efficacy and comparative efficacy of therapies for alcohol use disorder.
Data sources: PubMed, the Cochrane Library, the Cochrane Central Trials Registry, PsycINFO, CINAHL, and EMBASE were searched from November 2012 to September 9, 2022 Literature was subsequently systematically monitored to identify relevant articles up to August 14, 2023, and the PubMed search was updated on August 14, 2023.
Study selection: For efficacy outcomes, randomized clinical trials of at least 12 weeks' duration were included. For adverse effects, randomized clinical trials and prospective cohort studies that compared drug therapies and reported health outcomes or harms were included.
Data extraction and synthesis: Two reviewers evaluated each study, assessed risk of bias, and graded strength of evidence. Meta-analyses used random-effects models. Numbers needed to treat were calculated for medications with at least moderate strength of evidence for benefit.
Main outcomes and measures: The primary outcome was alcohol consumption. Secondary outcomes were motor vehicle crashes, injuries, quality of life, function, mortality, and harms.
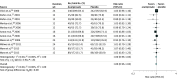
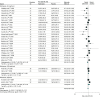
Results: Data from 118 clinical trials and 20 976 participants were included. The numbers needed to treat to prevent 1 person from returning to any drinking were 11 (95% CI, 1-32) for acamprosate and 18 (95% CI, 4-32) for oral naltrexone at a dose of 50 mg/d. Compared with placebo, oral naltrexone (50 mg/d) was associated with lower rates of return to heavy drinking, with a number needed to treat of 11 (95% CI, 5-41). Injectable naltrexone was associated with fewer drinking days over the 30-day treatment period (weighted mean difference, -4.99 days; 95% CI, -9.49 to -0.49 days) Adverse effects included higher gastrointestinal distress for acamprosate (diarrhea: risk ratio, 1.58; 95% CI, 1.27-1.97) and naltrexone (nausea: risk ratio, 1.73; 95% CI, 1.51-1.98; vomiting: risk ratio, 1.53; 95% CI, 1.23-1.91) compared with placebo.
Conclusions and relevance: In conjunction with psychosocial interventions, these findings support the use of oral naltrexone at 50 mg/d and acamprosate as first-line pharmacotherapies for alcohol use disorder.
Conflict of interest statement
Figures





Comment in
-
Amitriptyline for the Management of Irritable Bowel Syndrome in Primary Care.Gastroenterology. 2024 May;166(5):935-936. doi: 10.1053/j.gastro.2023.12.015. Epub 2023 Dec 24. Gastroenterology. 2024. PMID: 38147930 No abstract available.
-
Pharmacotherapy for Alcohol Use Disorder.JAMA. 2024 Mar 5;331(9):799-800. doi: 10.1001/jama.2023.28408. JAMA. 2024. PMID: 38441587 No abstract available.
References
-
- Centers for Disease Control and Prevention . Deaths from excessive alcohol use in the United States. Accessed January 23, 2023, https://www.cdc.gov/alcohol/features/excessive-alcohol-deaths.html
-
- Substance Abuse and Mental Health Services Administration . Key Substance Use and Mental Health Indicators in the United States: Results From the 2020 National Survey on Drug Use and Health. Published 2021. Accessed May 3, 2023. https://www.samhsa.gov/data/
-
- Substance Abuse and Mental Health Services Administration . National Survey on Drug Use and Health, detailed tables. Accessed July 25, 2022. https://www.samhsa.gov/data/report/2020-nsduh-detailed-tables
-
- Substance Abuse and Mental Health Services Administration . Key Substance Use and Mental Health Indicators in the United States: Results From the 2021 National Survey on Drug Use and Health. Published 2022. Accessed May 3, 2023. https://www.samhsa.gov/data/sites/default/files/reports/rpt39443/2021NSD...
-
- Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for Adults with Alcohol-Use Disorders in Outpatient Settings. Published May 13, 2014. Accessed May 3, 2023. https://effectivehealthcare.ahrq.gov/products/alcohol-misuse-drug-therap... - PubMed

