Platinum Nanozymes Counteract Photoreceptor Degeneration and Retina Inflammation in a Light-Damage Model of Age-Related Macular Degeneration
- PMID: 37934489
- PMCID: PMC10690844
- DOI: 10.1021/acsnano.3c07517
Platinum Nanozymes Counteract Photoreceptor Degeneration and Retina Inflammation in a Light-Damage Model of Age-Related Macular Degeneration
Abstract
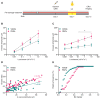
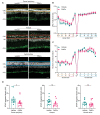
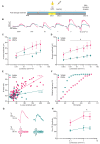
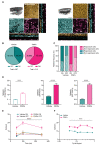
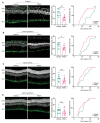
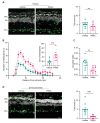
Degeneration of photoreceptors in age-related macular degeneration (AMD) is associated with oxidative stress due to the intense aerobic metabolism of rods and cones that if not properly counterbalanced by endogenous antioxidant mechanisms can precipitate photoreceptor degeneration. In spite of being a priority eye disease for its high incidence in the elderly, no effective treatments for AMD exist. While systemic administration of antioxidants has been unsuccessful in slowing down degeneration, locally administered rare-earth nanoparticles were shown to be effective in preventing retinal photo-oxidative damage. However, because of inherent problems of dispersion in biological media, limited antioxidant power, and short lifetimes, these NPs are still confined to the preclinical stage. Here we propose platinum nanoparticles (PtNPs), potent antioxidant nanozymes, as a therapeutic tool for AMD. PtNPs exhibit high catalytic activity at minimal concentrations and protect primary neurons against oxidative insults and the ensuing apoptosis. We tested the efficacy of intravitreally injected PtNPs in preventing or mitigating light damage produced in dark-reared albino Sprague-Dawley rats by in vivo electroretinography (ERG) and ex vivo retina morphology and electrophysiology. We found that both preventive and postlesional treatments with PtNPs increased the amplitude of ERG responses to light stimuli. Ex vivo recordings demonstrated the selective preservation of ON retinal ganglion cell responses to light stimulation in lesioned retinas treated with PtNPs. PtNPs administered after light damage significantly preserved the number of photoreceptors and inhibited the inflammatory response to degeneration, while the preventive treatment had a milder effect. The data indicate that PtNPs can effectively break the vicious cycle linking oxidative stress, degeneration, and inflammation by exerting antioxidant and anti-inflammatory actions. The increased photoreceptor survival and visual performances in degenerated retinas, together with their high biocompatibility, make PtNPs a potential strategy to cure AMD.
Keywords: Müller cells; electroretinogram; high-density multielectrode arrays; microglia; nanoparticles; oxidative stress; photoreceptor death.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

