Dabrafenib protects from cisplatin-induced hearing loss in a clinically relevant mouse model
- PMID: 37934596
- PMCID: PMC10807719
- DOI: 10.1172/jci.insight.171140
Dabrafenib protects from cisplatin-induced hearing loss in a clinically relevant mouse model
Abstract
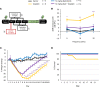
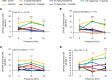
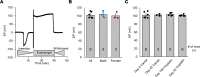
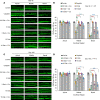
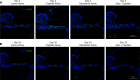
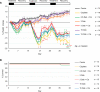
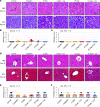
The widely used chemotherapy cisplatin causes permanent hearing loss in 40%-60% of patients with cancer. One drug, sodium thiosulfate, is approved by the FDA for use in pediatric patients with localized solid tumors for preventing cisplatin-induced hearing loss, but more drugs are desperately needed. Here, we tested dabrafenib, an FDA-approved BRAF kinase inhibitor and anticancer drug, in a clinically relevant multidose cisplatin mouse model. The protective effects of dabrafenib, given orally twice daily with cisplatin, were determined by functional hearing tests and cochlear outer hair cell counts. Toxicity of the drug cotreatment was evaluated, and levels of phosphorylated ERK were measured. A dabrafenib dose of 3 mg/kg BW, twice daily, in mice, was determined to be the minimum effective dose, and it is equivalent to one-tenth of the daily FDA-approved dose for human cancer treatment. The levels of hearing protection acquired, 20-25 dB at the 3 frequencies tested, in both female and male mice, persisted for 4 months after completion of treatments. Moreover, dabrafenib exhibited a good in vivo therapeutic index (> 25), protected hearing in 2 mouse strains, and diminished cisplatin-induced weight loss. This study demonstrates that dabrafenib is a promising candidate drug for protection from cisplatin-induced hearing loss.
Keywords: Cancer; Drug therapy; Otology; Protein kinases; Therapeutics.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

