Nucleation and Growth of Amyloid Fibrils
- PMID: 37934627
- PMCID: PMC11426289
- DOI: 10.1021/acs.jpcb.3c05300
Nucleation and Growth of Amyloid Fibrils
Abstract
The formation of amyloid fibrils is a complex phenomenon that remains poorly understood at the atomic scale. Herein, we perform extended unbiased all-atom simulations in explicit solvent of a short amphipathic peptide to shed light on the three mechanisms accounting for fibril formation, namely, nucleation via primary and secondary mechanisms, and fibril growth. We find that primary nucleation takes place via the formation of an intermediate state made of two laminated β-sheets oriented perpendicular to each other. The amyloid fibril spine subsequently emerges from the rotation of these β-sheets to account for peptides that are parallel to each other and perpendicular to the main axis of the fibril. Growth of this spine, in turn, takes place via a dock-and-lock mechanism. We find that peptides dock onto the fibril tip either from bulk solution or after diffusing on the fibril surface. The latter docking pathway contributes significantly to populate the fibril tip with peptides. We also find that side chain interactions drive the motion of peptides in the lock phase during growth, enabling them to adopt the structure imposed by the fibril tip with atomic fidelity. Conversely, the docked peptide becomes trapped in a local free energy minimum when docked-conformations are sampled randomly. Our simulations also highlight the role played by nonpolar fibril surface patches in catalyzing and orienting the formation of small cross-β structures. More broadly, our simulations provide important new insights into the pathways and interactions accounting for primary and secondary nucleation as well as the growth of amyloid fibrils.
Figures

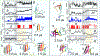


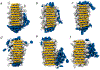
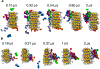


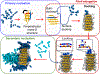
References
-
- Ilie IM; Caflisch A Simulation Studies of Amyloidogenic Polypeptides and Their Aggregates. Chemical Reviews 2019, 119, 6956–6993. - PubMed
-
- Nguyen PH; Ramamoorthy A; Sahoo BR; Zheng J; Faller P; Straub JE; Dominguez L; Shea J-E; Dokholyan NV; De Simone A; others Amyloid Oligomers: A Joint Experimental/Computational Perspective on Alzheimer’s Disease, Parkinson’s Disease, Type II Diabetes, and Amyotrophic Lateral Sclerosis. Chemical Reviews 2021, 121, 2545–2647. - PMC - PubMed
-
- Owen MC; Gnutt D; Gao M; Wärmländer SKTS; Jarvet J; Gräslund A; Winter R; Ebbinghaus S; Strodel B Effects of in Vivo Conditions on Amyloid Aggregation. Chemical Society Reviews 2019, 48, 3946–3996. - PubMed
-
- Cao Y; Tang X; Yuan M; Han W Computational studies of protein aggregation mediated by amyloid: fibril elongation and secondary nucleation. Progress in Molecular Biology and Translational Science 2020, 170, 461–504. - PubMed
-
- Morriss-Andrews A; Shea J-E Simulations of Protein Aggregation: Insights from Atomistic and Coarse-Grained Models. The Journal of Physical Chemistry Letters 2014, 5, 1899–1908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

