Cystic fibrosis screening, evaluation, and management of hepatobiliary disease consensus recommendations
- PMID: 37934656
- PMCID: PMC11020118
- DOI: 10.1097/HEP.0000000000000646
Cystic fibrosis screening, evaluation, and management of hepatobiliary disease consensus recommendations
Abstract
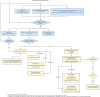
Cystic fibrosis (CF) may cause a spectrum of hepatobiliary complications, including portal hypertension, multilobular cirrhosis, and liver failure. Current guidelines on the detection and monitoring of hepatobiliary complications in CF were published in 1999. The CF Foundation assembled a committee to evaluate research advances and formulate revised guidelines for CF-associated liver disease. A committee of hepatologists, gastroenterologists, pulmonologists, pharmacists, nurses, dietitians, individuals with CF, and the parents of a child with CF devised "population, intervention, comparison, and outcome" questions regarding hepatobiliary disease in CF. PubMed literature searches were performed for each population, intervention, comparison, and outcome question. Recommendations were voted on with 80% agreement required to approve a recommendation. Public comment on initial recommendations was solicited prior to the formulation of final recommendations. Thirty-one population, intervention, comparison, and outcome questions were assembled, 6401 manuscripts were title screened for relevance, with 1053 manuscripts undergoing detailed full-text review. Seven recommendations were approved for screening, 13 for monitoring of existing disease, and 14 for treatment of CF-associated hepatobiliary involvement or advanced liver disease. One recommendation on liver biopsy did not meet the 80% threshold. One recommendation on screening ultrasound was revised and re-voted on. Through a multidisciplinary committee and public engagement, we have assembled updated recommendations and guidance on screening, monitoring, and treatment of CF-associated hepatobiliary involvement and advanced liver disease. While research gaps remain, we anticipate that these recommendations will lead to improvements in CF outcomes through earlier detection and increased evidence-based approaches to monitoring and treatment.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Zachary M. Sellers Consults for AbbVie, Anionix, Renexxion, and Vertex. He received grants from the Cystic Fibrosis Foundation. Meghana Sathe consults for Nestle received grants from Anagram Therapeutics and the Cystic Fibrosis Foundation. She has other interests in Alcresta Therapeutics. Dominique Debray consults and received grants from Vertex. She consults for Alexion, Mirum, Orphalan, and Univar. Simon C. Ling consults and received grants from AbbVie. He consults for Medison and Mirium. Daniel Peckham is on the speakers’ bureau for Vertex. Kay Vavrina is on the speakers’ bureau for AbbVie and Alcresta. Michael R. Narkewicz consults for Vertex He received grants from AbbVie, the Cystic Fibrosis Foundation, and Gilead. The remaining authors have no conflicts to report.
Figures

Comment in
-
New guidelines for screening, evaluation and management of hepatobiliary disease in cystic fibrosis.Hepatobiliary Surg Nutr. 2024 Oct 1;13(5):875-878. doi: 10.21037/hbsn-24-442. Epub 2024 Sep 26. Hepatobiliary Surg Nutr. 2024. PMID: 39507715 Free PMC article. No abstract available.
-
New insights on portal hypertension's screening in people with cystic fibrosis.Hepatobiliary Surg Nutr. 2024 Oct 1;13(5):894-897. doi: 10.21037/hbsn-24-467. Epub 2024 Sep 26. Hepatobiliary Surg Nutr. 2024. PMID: 39507743 Free PMC article. No abstract available.
References
-
- Leung DH, Narkewicz MR. Cystic fibrosis-related cirrhosis. J Cyst Fibros. 2017;16(suppl 2):S50–61. - PubMed
-
- Sokol RJ, Durie PR. Recommendations for management of liver and biliary tract disease in cystic fibrosis. Cystic Fibrosis Foundation Hepatobiliary Disease Consensus Group. J Pediatr Gastroenterol Nutr. 1999;28(suppl 1):S1–13. - PubMed
-
- Woodruff SA, Sontag MK, Accurso FJ, Sokol RJ, Narkewicz MR. Prevalence of elevated liver enzymes in children with cystic fibrosis diagnosed by newborn screen. J Cyst Fibros. 2017;16:139–45. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

