One-year safety and efficacy of mitapivat in sickle cell disease: follow-up results of a phase 2, open-label study
- PMID: 37934880
- PMCID: PMC10761354
- DOI: 10.1182/bloodadvances.2023011477
One-year safety and efficacy of mitapivat in sickle cell disease: follow-up results of a phase 2, open-label study
Abstract
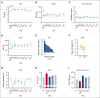
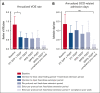
Targeting the primary pathogenic event of sickle cell disease (SCD), the polymerization of sickle hemoglobin (HbS), may prevent downstream clinical events. Mitapivat, an oral pyruvate kinase (PK) activator, has therapeutic potential by increasing adenosine triphosphate (ATP) and decreasing 2,3-diphosphoglycerate (2,3-DPG), a glycolytic red blood cell (RBC) intermediate. In the previously reported 8-week dose-finding period of this phase 2, investigator-initiated, open-label study, mitapivat was well tolerated and showed efficacy in SCD. Here, the 1-year fixed-dose extension period is reported in which 9 of 10 included patients (90%) aged ≥16 years with SCD (HbSS, HbS/β0, or HbS/β+) continued with mitapivat. Mostly mild treatment-emergent adverse events (AEs) (most commonly, transaminase increase and headache) were still reported. Apart from the reported nontreatment-related serious AE (SAE) of a urinary tract infection in the dose-finding period, 1 nontreatment-related SAE occurred in the fixed-dose extension period in a patient who died of massive pulmonary embolism due to COVID-19. Importantly, sustained improvement in Hb level (mean increase, 1.1 ± 0.7 g/dL; P = .0014) was seen, which was accompanied by decreases in markers of hemolysis. In addition, the annualized rate of vaso-occlusive events reduced significantly from a historic baseline of 1.33 ± 1.32 to 0.64 ± 0.87 (P = .0489) when combining the dose-finding period and fixed-dose extension period. Cellularly, the ATP:2,3-DPG ratio and Hb-oxygen affinity significantly increased and RBC sickling (point of sickling) nonsignificantly reduced. Overall, this study demonstrated 1-year safety and efficacy of treatment with mitapivat in SCD, supporting further evaluation in ongoing phase 2/3 study (RISE UP, NCT05031780). This trial was registered at https://www.clinicaltrialsregister.eu/ as NL8517 and EudraCT 2019-003438-18.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.A.E.R. and R.v.W. receive research funding from Axcella Therapeutics and Pfizer. M.A.E.R., R.v.W., and E.J.v.B. receive research funding from and are consultants for Agios Pharmaceuticals Inc. R.v.W. and E.J.v.B. are consultants for Pfizer. M.H.C. (institution) has received investigator-initiated research and travel grants as well as speaker fees over the years from the Netherlands Organisation for Scientific Research and Netherlands National Research Agenda, the Netherlands Organization for Health Research and Development (ZonMw), the Dutch Innovatiefonds Zorgverzekeraars, Stichting Haemophilia, Baxter/Baxalta/Shire/Takeda, Pfizer, Bayer Schering Pharma, CSL Behring, Sobi Biogen, Novo Nordisk, Novartis, Roche, and Nordic Pharma,; and has served as a steering board member for Roche, Bayer, and Novartis. E.N. receives research funding from Novartis and Emmaus and participates in advisory board of Novartis. B.J.B. receives research funding from Sanquin, Pfizer, and Novartis; and has participated in the advisory boards of Novartis, Global Blood Therapeutics /Pfizer, Novo Nordisk, Celgene, Chiesi, CSL Behring, and bluebird bio. R.E.G.S. has received research funding and/or speaker fees from Bayer, CSL Behring, Hemab, NovoNordisk, Octapharma, Sanofi, and Sobi (all paid to institution). The remaining authors declare no competing financial interests.
Figures



References
-
- Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311–323. - PubMed
-
- Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561–1573. - PubMed
-
- World Health Assembly 59 . World Health Organization; 2006. Sickle-Cell Anaemia: Report by the Secretariat.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

