Real-world effectiveness and safety of RC48-ADC alone or in combination with PD-1 inhibitors for patients with locally advanced or metastatic urothelial carcinoma: A multicenter, retrospective clinical study
- PMID: 37935113
- PMCID: PMC10726858
- DOI: 10.1002/cam4.6680
Real-world effectiveness and safety of RC48-ADC alone or in combination with PD-1 inhibitors for patients with locally advanced or metastatic urothelial carcinoma: A multicenter, retrospective clinical study
Abstract
Introduction: Previous RC48 (Disitamab Vedotin) studies established that the safety and efficacy of RC48-antibody-drug conjugate (ADC), either alone or combined with toripalimab, for metastatic urothelial carcinoma (mUC) patients exhibiting human epidermal growth factor receptor 2 (HER2)-positive or even HER2-negative status after standard chemotherapy failure.
Methods: With locally advanced or metastatic urothelial carcinoma (la/mUC), patients who received RC48-ADC monotherapy or a combination with programmed cell death protein 1 (PD-1) inhibitors between August 2021 and October 2022 were enrolled in this retrospective observational study to evaluate the real-world antitumor effectiveness and safety.
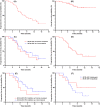
Results: Among the 38 enrolled patients (29 males; median age 67.5 years [38-93]), 8 received RC48-ADC monotherapy, while 30 received combination therapy. Initially, 63.2% (24/38) of the patients had received ≥1 line of prior treatment, and 63.2% (24/38) had visceral metastasis. UC of the bladder represented the majority type in 68.4% (26/38) of cases. By the data cutoff in March 2023, the overall objective response rate (ORR) was 63.2% (95% CI, 47.1%-79.2%), with a disease control rate (DCR) of 89.5% (95% CI, 79.3%-99.7%). Median follow-up time was 10.6 months. The median progression-free survival (PFS) was 8.2 months (95% CI, 5.9-10.5), with a 6-month PFS rate of 63.2% and a 12-month PFS rate of 34.1%. Median overall survival (OS) was not reached, with a 12-month OS rate of 76.7%. The median duration of response was 7.3 months (95% CI, 4.6-10.0) among 24 patients evaluated as partial response (PR). The most common treatment-related adverse events (TRAEs) included anemia (71.1%), anorexia (57.9%), asthenia (52.6%), hypoesthesia (52.6%), bone marrow suppression (47.4%), alopecia (47.4%), nausea (44.7%), proteinuria (36.8%), vomiting (34.2%), and hypoalbuminemia (31.6%). No patient experienced TRAEs of Grade ≥3. One patient had an immune-related adverse event (irAE) of rash related to toripalimab.
Conclusions: Both as monotherapy and in combination with PD-1 inhibitors, RC48-ADC exhibits promising effectiveness and manageable safety profile for mUC patients in real-world settings.
Keywords: antibody-drug conjugate; disitamab vedotin; metastatic urothelial carcinoma; real-world study.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors indicate no financial or non‐financial interests.
Figures

References
-
- von der Maase H, Sengelov L, Roberts JT, et al. Long‐term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602‐4608. - PubMed
-
- Joaquim B, Hans vM, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC intergroup study 30987. J Clin Oncol. 2012;30(10):1107‐1113. - PMC - PubMed
-
- Xinan S, Haige C, Bin H, et al. Safety, efficacy and biomarker analysis of Toripalimab in patients with previously treated advanced urothelial carcinoma: results from a multicenter phase II trial POLARIS‐03. Clinical cancer research: an official journal of the American association for. Cancer Res. 2022;28(3):489‐497. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

