Feasibility and impact of ketogenic dietary interventions in polycystic kidney disease: KETO-ADPKD-a randomized controlled trial
- PMID: 37935200
- PMCID: PMC10694658
- DOI: 10.1016/j.xcrm.2023.101283
Feasibility and impact of ketogenic dietary interventions in polycystic kidney disease: KETO-ADPKD-a randomized controlled trial
Abstract
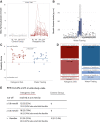
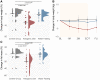
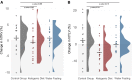
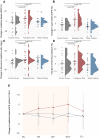
Ketogenic dietary interventions (KDIs) are beneficial in animal models of autosomal-dominant polycystic kidney disease (ADPKD). KETO-ADPKD, an exploratory, randomized, controlled trial, is intended to provide clinical translation of these findings (NCT04680780). Sixty-six patients were randomized to a KDI arm (ketogenic diet [KD] or water fasting [WF]) or the control group. Both interventions induce significant ketogenesis on the basis of blood and breath acetone measurements. Ninety-five percent (KD) and 85% (WF) report the diet as feasible. KD leads to significant reductions in body fat and liver volume. Additionally, KD is associated with reduced kidney volume (not reaching statistical significance). Interestingly, the KD group exhibits improved kidney function at the end of treatment, while the control and WF groups show a progressive decline, as is typical in ADPKD. Safety-relevant events are largely mild, expected (initial flu-like symptoms associated with KD), and transient. Safety assessment is complemented by nuclear magnetic resonance (NMR) lipid profile analyses.
Keywords: ADPKD; cholesterol; ketogenic diet; ketosis; kidney; lipids; low carb; nutrition; polycystic kidney disease; water fasting.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests R.-U.M. is a member of the scientific advisory board of Santa Barbara Nutrients and chair of the working group “Genes&Kidney” of the European Renal Association (ERA). T.W. is an inventor on issued and pending patents filed by the University of California, Santa Barbara related to the topic of this article. T.W. is a shareholder of Santa Barbara Nutrients, Inc., and holds a managerial position. T.W. is a scientific advisor and shareholder of Chinook Therapeutics and received research funding from Chinook Therapeutics. The Department II of Internal Medicine (University Hospital Cologne) received research funding from Otsuka Pharmaceuticals not directly related to the study at hand.
Figures