Insights into the structure and function of the RNA ligase RtcB
- PMID: 37935993
- PMCID: PMC10630183
- DOI: 10.1007/s00018-023-05001-5
Insights into the structure and function of the RNA ligase RtcB
Abstract
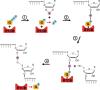
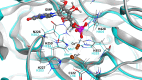
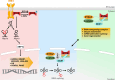
To be functional, some RNAs require a processing step involving splicing events. Each splicing event necessitates an RNA ligation step. RNA ligation is a process that can be achieved with various intermediaries such as self-catalysing RNAs, 5'-3' and 3'-5' RNA ligases. While several types of RNA ligation mechanisms occur in human, RtcB is the only 3'-5' RNA ligase identified in human cells to date. RtcB RNA ligation activity is well known to be essential for the splicing of XBP1, an essential transcription factor of the unfolded protein response; as well as for the maturation of specific intron-containing tRNAs. As such, RtcB is a core factor in protein synthesis and homeostasis. Taking advantage of the high homology between RtcB orthologues in archaea, bacteria and eukaryotes, this review will provide an introduction to the structure of RtcB and the mechanism of 3'-5' RNA ligation. This analysis is followed by a description of the mechanisms regulating RtcB activity and localisation, its known partners and its various functions from bacteria to human with a specific focus on human cancer.
Keywords: 3′–5′ RNA ligase; Archease; Cancer; Crystal structure; RNA 2′,3′-cyclic phosphate and 5′-OH ligase [RtcB]; RNA ligation; Transfer RNA [tRNA]; Unfolded protein response [UPR]; X-box-binding protein 1 [XBP1]; tRNA ligase complex.
© 2023. The Author(s).
Conflict of interest statement
AG, AS and LAE are cofounders of Cell Stress Discoveries. LAE is cofounder of ANYO Labs.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

