The correlation between gut microbiome and atrial fibrillation: pathophysiology and therapeutic perspectives
- PMID: 37936201
- PMCID: PMC10629124
- DOI: 10.1186/s40779-023-00489-1
The correlation between gut microbiome and atrial fibrillation: pathophysiology and therapeutic perspectives
Abstract
Regulation of gut microbiota and its impact on human health is the theme of intensive research. The incidence and prevalence of atrial fibrillation (AF) are continuously escalating as the global population ages and chronic disease survival rates increase; however, the mechanisms are not entirely clarified. It is gaining awareness that alterations in the assembly, structure, and dynamics of gut microbiota are intimately engaged in the AF progression. Owing to advancements in next-generation sequencing technologies and computational strategies, researchers can explore novel linkages with the genomes, transcriptomes, proteomes, and metabolomes through parallel meta-omics approaches, rendering a panoramic view of the culture-independent microbial investigation. In this review, we summarized the evidence for a bidirectional correlation between AF and the gut microbiome. Furthermore, we proposed the concept of "gut-immune-heart" axis and addressed the direct and indirect causal roots between the gut microbiome and AF. The intricate relationship was unveiled to generate innovative microbiota-based preventive and therapeutic interventions, which shed light on a definite direction for future experiments.
Keywords: Atrial fibrillation (AF); Gut microbiome; Immunity; Meta-omics; Metabolites.
© 2023. The Author(s).
Conflict of interest statement
All authors declare that they have no competing interests.
Figures
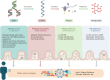

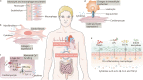
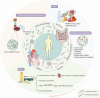
References
Publication types
MeSH terms
Grants and funding
- 2022YFC2303100/The National Key Research and Development Program of China
- JNL-2022001A/Research Project of Jinan Microecological Biomedicine Shandong Laboratory
- JNL-2022015B/Research Project of Jinan Microecological Biomedicine Shandong Laboratory
- HNSWJW-2022013/Central Plains Talent Program-Central Plains Youth Top Talents, Young and Middle-aged Academic Leaders of Henan Provincial Health Commission
- QNCXTD2023002/Funding for Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University
LinkOut - more resources
Full Text Sources
Medical

