Inhibition of ATR opposes glioblastoma invasion through disruption of cytoskeletal networks and integrin internalization via macropinocytosis
- PMID: 37936324
- PMCID: PMC10995506
- DOI: 10.1093/neuonc/noad210
Inhibition of ATR opposes glioblastoma invasion through disruption of cytoskeletal networks and integrin internalization via macropinocytosis
Abstract
Background: Glioblastomas have highly infiltrative growth patterns that contribute to recurrence and poor survival. Despite infiltration being a critical therapeutic target, no clinically useful therapies exist that counter glioblastoma invasion. Here, we report that inhibition of ataxia telangiectasia and Rad 3 related kinase (ATR) reduces invasion of glioblastoma cells through dysregulation of cytoskeletal networks and subsequent integrin trafficking.
Methods: Glioblastoma motility and invasion were assessed in vitro and in vivo in response to ATR inhibition (ATRi) and ATR overexpression using time-lapse microscopy, two orthotopic glioblastoma models, and intravital imaging. Disruption to cytoskeleton networks and endocytic processing were investigated via high-throughput, super-resolution and intravital imaging.
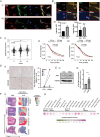
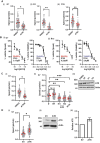
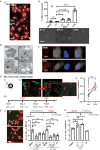
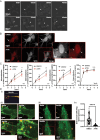
Results: High ATR expression was associated with significantly poorer survival in clinical datasets while histological, protein expression, and spatial transcriptomics using glioblastoma tumor specimens revealed higher ATR expression at infiltrative margins. Pharmacological inhibition with two different compounds and RNAi targeting of ATR opposed the invasion of glioblastoma, whereas overexpression of ATR drove migration. Subsequent investigation revealed that cytoskeletal dysregulation reduced macropinocytotic internalization of integrins at growth-cone-like structures, resulting in a tumor microtube retraction defect. The biological relevance and translational potential of these findings were confirmed using two orthotopic in vivo models of glioblastoma and intravital imaging.
Conclusions: We demonstrate a novel role for ATR in determining invasion in glioblastoma cells and propose that pharmacological targeting of ATR could have far-reaching clinical benefits beyond radiosensitization.
Keywords: ATR; DNA damage response; glioblastoma; high-grade glioma; invasion; macropinocytosis.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
None declared.
Figures



References
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Walker MD, AlexanderE, Jr, Hunt WE, et al. . Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas A cooperative clinical trial. J Neurosurg. 1978;49(3):333–343. - PubMed
-
- Birch JL, Strathdee K, Gilmour L, et al. . A novel small-molecule inhibitor of MRCK prevents radiation-driven invasion in glioblastoma. Cancer Res. 2018;78(22):6509–6522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 23983/CRUK_/Cancer Research UK/United Kingdom
- A31287/CRUK_/Cancer Research UK/United Kingdom
- NC/P001335/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- 29802/CRUK_/Cancer Research UK/United Kingdom
- MR/T04358X/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Miscellaneous

